Abstract
Background and aims
Enhanced aluminum (Al) resistance has been observed in dicots over-expressing enzymes involved in organic acid synthesis; however, this approach for improving Al resistance has not been investigated in monocots. Among the cereals, oat (Avena sativa L.) is considered to be Al resistant, but the basis of resistance is not known.
Methods
A hydroponic assay and hematoxylin staining for Al accumulation in roots were used to evaluate Al resistance in 15 oat cultivars. Malate and citrate release from roots was measured over a 24 h period. A malate dehydrogenase gene, neMDH, from alfalfa (Medicago sativa L.) was used to transform oat.
Results
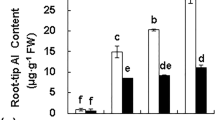
Oat seedlings were highly resistant to Al, as a concentration of 325 μM AlK(SO4)2 was needed to cause a 50% decrease in root growth. Most oat cultivars tested are naturally resistant to high concentrations of Al and effectively excluded Al from roots. Al-dependent release of malate and Al-independent release of citrate was observed. Al resistance was enhanced in a transgenic oat line with the highest accumulation of neMDH protein. However, overall root growth of this line was reduced and expression of neMDH in transgenic oat did not enhance malate secretion.
Conclusions
Release of malate from oat roots was associated with Al resistance, which suggests that malate plays a role in Al resistance of oat. Over-expression of alfalfa neMDH enhanced Al resistance in some lines but was not effective alone for crop improvement.








Similar content being viewed by others
References
Al-Saady NA, Torbert KA, Smith L, Makarevitch I, Baldridge G, Zeyen RJ, Muelbauer GJ, Olszewski NE, Somers DA (2004) Tissue specificity of the sugarcane bacilliform virus promoter in oat, barley and wheat. Mol Breed 14:331–338
Andersson M (1988) Toxicity and tolerance of aluminum in vascular plants. Water Air Soil Pollut 39:439–462
Anoop VM, Bas U, McCammon MT, McAlister-Henn L, Taylor GJ (2003) Modulation of citrate metabolism alters aluminum tolerance in yeast and transgenic canola overexpressing a mitrochondrial citrate synthase. Plant Physiol 132:2205–2217
Archambault DJ, Zhang G, Taylor GJ (1996) Accumulation of Al in root mucilage of an Al-resistant and an Al-sensitive cultivar of wheat. Plant Physiol 112:1471–1478
Barone P, Rosellini D, LaFayette P, Bouton J, Veronesi F, Parrott W (2008) Bacterial citrate synthase expression and soil aluminum tolerance in transgenic alfalfa. Plant Cell Rep 27:893–901
Canaado GMA, Loguercio LL, Martins PR, Parentoni SN, Paiva E, Borém A, Lopes MA (1999) Hematoxylin staining as a phenotypic index for aluminum tolerance selection in tropical maize (Zea mays L.). Theor Appl Genet 99:747–754
Castilhos G, Farias JG, de Bernardi SA, de Oliveira PH, Nicoloso FT, Schetinger MRC, Delatorre CA (2011) Aluminum-stress response in oat genotypes with monogenic tolerance. Environ Exp Bot. doi:10.1016/j.envexpbot.2011.05.007
Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ (1993a) Aluminum tolerance in wheat (Triticum aestivum L.). I. Uptake and distribution of aluminum in root apices. Plant Physiol 103:685–693
Delhaize E, Hebb DM, Ryan PR (2001) Expression of a Pseudomonas aeruginosa citrate synthase gene in tobacco is not associated with either enhanced citrate accumulation or efflux. Plant Physiol 125:2059–2067
Delhaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matusmoto H (2004) Engineering high-level aluminum tolerance in barley with the ALMT1gene. Proc Natl Acad Sci U S A 101:15249–15254
Delhaize E, Ryan PR, Randall PJ (1993b) Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol 103:695–702
Foy CD, Smith DH, Briggle LW (1987) Tolerances of oat cultivars to and acid soil high in exchangeable aluminum. J Plant Nutr 10:1163–1174
Fromm ME, Taylor LP, Walbot V (1986) Stable transformation of maize after gene transfer by electroporation. Nature 319:791–793
Hayes JE, Ma JF (2003) Al-induced efflux of organic acid anions is poorly associated with internal organic acid metabolism in triticale roots. J Exp Bot 54:1753–1759
Kinraide TB (1991) Identity of the rhizotoxic aluminium species. In: Wright RJ, Baligar VC, Murrmann RP (eds) Plant-soil interactions at low pH. Dordrecht, Boston, pp 717–728
Kinraide TB (1997) Reconsidering the rhizotoxicity of hydroxyl, sulphate, and fluoride complexes of aluminum. J Exp Bot 48:1115–1124
Kochian LV, Piñeros MA, Hoekenga OA (2005) The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274:175–195
Koyama H, Kawamura A, Kihara T, Hara T, Takita E, Shibata A (2000) Overexpression of mitochondrial citrate synthase in Arabidopsis thaliana improved growth on a phosphorus-limited soil. Plant Cell Physiol 41:1030–1037
Lambers H, Chapin FS, Pons TL (1998) Plant physiological ecology. Springer, New York
Larsen PB, Tai CH, Kochian LV, Howell SH (1996) Arabidopsis mutants with increased sensitivity to aluminum. Plant Physiol 110:743–751
Li XF, Ma JF, Hiradate S, Matsumoto H (2000) Mucilage strongly binds aluminum but does not prevent roots from aluminum injury in Zea mays. Physiol Plant 108:152–160
Maron LG, Piñeros MA, Guimarães CT, Magalhaes JV, Pleiman JK, Mao C, Shaff Jm Belicuas SNJ, Kochian LV (2010) Two functionally distinct members of the MATE (multi-drugand toxic compound extruxion) family of transporters potentially underlie two major aluminum tolerance QTLs in maize. Plant J 61:728–740
Miller SS, Driscoll BT, Gregerson RG, Gantt JS, Vance CP (1998) Alfalfa malate dehydrogenase (MDH): molecular cloning and characterization of five different forms reveals a unique nodule-enhanced MDH. Plant J 15:173–184
Miyasaka SC, Hawes MC (2001) Possible role of root border cells in detection and avoidance of aluminum toxicity. Plant Physiol 125:1978–1987
Nava IC, Delatorre CA, de Lima Duarte IT, Pacheco MT, Federizze LC (2006) Inheritance of aluminum tolerance and its effects on grain yield and grain quality in oats (Avena sativa L.). Euphytica 148:353–358
Ninamango-Cárdenas FE, Guimarães CT, Martins PR, Parentoni SN, Carneiro NP, Lopes MA, Moro JR, Paiva E (2003) Mapping QTLs for aluminum tolerance in maize. Euphytica 130:223–232
Pereira JF, Zhou G, Delhaize E, Richardson T, Zhou M, Ryan PR (2010) Engineering greater aluminum resistance in wheat by over-expressing TaALMT1. Ann Bot 106:205–214
Piñeros MA, Shaff JE, Manslank HS, Alves VMC, Kochian LV (2005) Aluminum resistance in maize cannot be solely explained by root organic acid exudation. A comparative physiological study. Plant Physiol 137:231–241
Polle E, Konzak CF, Kittrick JA (1978) Visual detection of aluminum tolerance levels in wheat by hematoxylin staining of seedling roots. Crop Sci 18:823–827
Ryan PR, Delhaize E (2010) The convergent evolution of aluminium resistance in plants exploits a convenient currency. Funct Plant Biol 37:275–284
Ryan P, Delhaize E, Randall P (1995) Characterization of Al-stimulated efflux of malate from the apices of Al-tolerant wheat lines. Planta 196:103–110
Ryan PR, Raman H, Gupta S, Horst WJ, Delhazie E (2009) A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiol 149:340–351
SAS Institute (2003) Version 9.1 ed. SAS Inst, Cary
Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum-activating transporter. Plant J 37:645–653
Shaff JE, Schultz BA, Craft EJ, Clark RT, Kochian LV (2010) GOECHEM-EZ: a chemical speciation program with greater power and flexibility. Plant Soil 330:207–214
Tamás L, Budíková S, Huttová J, Mistrík I, Šimonovičová M, Široká B (2005) Aluminum-induced cell death of barley-root border cells is correlated with peroxidase- and oxalate oxidase-mediated hydrogen peroxide production. Plant Cell Rep 24:189–194
Tarkalson DD, Payero JO, Hergert GW, Cassman KG (2006) Acidification of soil in a dry land winter wheat-sorghum/corn-fallow rotation in the semiarid U.S. Great Plains. Plant Soil 283:367–379
Tesfaye M, Temple SJ, Allan DL, Vance CP, Samac DA (2001) Overexpression of malate dehydrogenase in transgenic alfalfa enhances organic acid synthesis and confers tolerance to aluminum. Plant Physiol 127:1836–1844
Torbert KA, Rines HW, Kaeppler HF, Menon GR, Somers DA (1998) Genetically engineering elite oat cultivars. Crop Sci 38:1685–1687
Tzafrir I, Torbert KA, Lockhart BEL, Somers DA, Olszewski NE (1998) The sugarcane bacilliform badnavirus promoter is active in both monocots and dicots. Plant Mol Biol 38:347–356
von Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. Plant Soil 171:1–15
Wheeler DM, Edmeades DC, Christie RA, Gardner R (1992) Effect of aluminium on growth of 34 plant species: a summary of results obtained in low ionic strength solution culture. Plant Soil 146:61–66
Wight CP, Kibite S, Tinker NA, Molnar SJ (2006) Identification of molecular markers for aluminium tolerance in diploid oat through comparative mapping and QTL analysis. Theor Appl Genet 112:222–231
Yamaji N, Huang CF, Nagao S, Yano M, Sato Y, Nagamura Y, Ma JF (2009) A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell 21:3339–3349
Zheng SJ, Ma JF, Matsumoto H (1998) Continuous secretion of organic acids is related to aluminum resistance during relatively long-term exposure to aluminum stress. Physiol Plant 103:209–214
Zhu M, Ahn S, Matsumoto H (2003) Inhibition of growth and development of root border cells in wheat by Al. Physiol Plant 117:359–367
Acknowledgements
Mention of any trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U. S. Department of Agriculture. This paper is a joint contribution from the Plant Science Research Unit, USDA-ARS, and the Minnesota Agricultural Experiment Station. We gratefully acknowledge the assistance of Kim Torbert for production of transformed oat plants, Deon Stuthman and Roger Caspars for oat seed, and Karen Hilburn for assistance with lyophilization.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
About this article
Cite this article
Radmer, L., Tesfaye, M., Somers, D.A. et al. Aluminum resistance mechanisms in oat (Avena sativa L.). Plant Soil 351, 121–134 (2012). https://doi.org/10.1007/s11104-011-0937-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0937-1