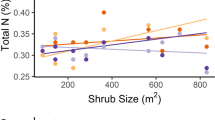
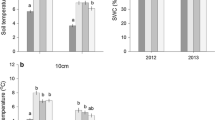
Abstract
Belowground processes and associated plant–microbial interactions play a critical role in how ecosystems respond to environmental change. However, the mechanisms and factors controlling processes such as soil carbon turnover can be difficult to quantify due to methodological or logistical constraints. Soil incubation experiments have the potential to greatly improve our understanding of belowground carbon dynamics, but relating results from laboratory-based incubations to processes measured in the field is challenging. This study has two goals: (1) development of a hierarchical Bayesian (HB) model for analyzing soil incubation data and complementary field data to gain a more mechanistic understanding of soil carbon turnover; (2) application of the approach to soil incubation data collected from a semi-arid riparian grassland experiencing encroachment by nitrogen-fixing shrubs (mesquite). Soil was collected from several depths beneath large-sized shrubs, medium-sized shrubs, grass, and bare ground—the four primary microsite-types found in this ecosystem. We measured respiration rates from substrate-induced incubations, which were accompanied by measurements of soil microbial biomass, soil carbon, and soil nitrogen. Soils under large shrubs had higher respiration rates and support 2.0, 1.9, and 2.6 times greater soil carbon, microbial biomass, and microbial carbon-use efficiency, respectively, compared to soils in grass microsites. The effect of large shrubs on these components is most pronounced near the soil surface where microbial carbon-use efficiency is high because of enhanced litter quality. Grass microsites were very similar to bare ground in many aspects (carbon content, microbial biomass, etc.). Encroachment of mesquite shrubs into this semi-arid grassland may enhance carbon and nutrient cycling and increase the spatial heterogeneity of soil resource pools and fluxes. The HB approach allowed us to synthesize diverse data sources to identify the potential mechanisms of soil carbon and microbial change associated with shrub encroachment.






Similar content being viewed by others
References
Alvarez R, Alvarez CR (2000) Soil organic matter pools and their associations with carbon mineralization kinetics. Soil Sci Soc Am J 64:184–189
Archer S, Schimel DS, Holland EA (1995) Mechanisms of shrubland expansion—land-use, climate or CO2. Clim Change 29:91–99 doi:10.1007/BF01091640
Austin AT, Yahdjian L, Stark JM et al (2004) Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141:221–235 doi:10.1007/s00442-004-1519-1
Ball AS (1997) Microbial decomposition at elevated CO2 levels: effect of litter quality. Glob Chang Biol 3:379–386 doi:10.1046/j.1365-2486.1997.t01-1-00089.x
Berliner M (1996) Hierarchical Bayesian time series models. In: Hanson K, Silver R (eds) Maximum entropy and Bayesian methods. Kluwer, Norwell, MA, pp 15–22
Brooks SP, Gelman A (1998) General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 7:434–455 doi:10.2307/1390675
Chapin FS, Shaver GR, Giblin AE et al (1995) Responses of arctic tundra to experimental and observed changes in climate. Ecology 76:694–711 doi:10.2307/1939337
Clark JS (2005) Why environmental scientists are becoming Bayesians. Ecol Lett 8:2–14 doi:10.1111/j.1461-0248.2004.00702.x
Dalias P, Anderson JM, Bottner P et al (2001) Long-term effects of temperature on carbon mineralisation processes. Soil Biol Biochem 33:1049–1057 doi:10.1016/S0038-0717(01)00009-8
Dellaportas P, Stephens A (1995) Bayesian analysis of errors-in-variables regression models. Biometrics 51:1085–1095 doi:10.2307/2533007
Dutta K, Schuur EAG, Neff JC et al (2006) Potential carbon release from permafrost soils of Northeastern Siberia. Glob Chang Biol 12:2336–2351 doi:10.1111/j.1365-2486.2006.01259.x
Fierer N, Schimel JP, Holden PA (2003) Influence of drying-rewetting frequency on soil bacterial community structure. Microb Ecol 45:63–71 doi:10.1007/s00248-002-1007-2
Fierer N, Colman BP, Schimel JP et al (2006) Predicting the temperature dependence of microbial respiration in soil: a continental-scale analysis. Glob Biogeochem Cycles 20:10
Fliessbach A, Sarig S, Steinberger Y (1994) Effects of water pulses and climatic conditions on microbial biomass kinetics and microbial activity in a yermosol of the central Negev. Arid Soil Res Rehabil 8:353–362
Gelman A, Carlin JB, Stern HS et al (2004) Bayesian data analysis. CRC, Boca Raton, p 668
Grandy AS, Robertson GP (2007) Land-use intensity effects on soil organic carbon accumulation rates and mechanisms. Ecosystems (N Y, Print) 10:58–73 doi:10.1007/s10021-006-9010-y
Hamer U, Marschner B (2005) Priming effects in soils after combined and repeated substrate additions. Geoderma 128:38–51 doi:10.1016/j.geoderma.2004.12.014
Hibbard KA, Archer S, Schimel DS et al (2001) Biogeochemical changes accompanying woody plant encroachment in a subtropical savanna. Ecology 82:1999–2011
Hook PB, Burke IC (2000) Biogeochemistry in a shortgrass landscape: control by topography, soil texture, and microclimate. Ecology 81:2686–2703
Houghton RA, Davidson EA, Woodwell GM (1998) Missing sinks, feedbacks, and understanding the role of terrestrial ecosystems in the global carbon balance. Glob Biogeochem Cycles 12:25–34 doi:10.1029/97GB02729
Hunt HW, Wall DH (2002) Modelling the effects of loss of soil biodiversity on ecosystem function. Glob Chang Biol 8:33–50 doi:10.1046/j.1365-2486.2002.00425.x
Huxman TE, Cable JM, Ignace DD et al (2004) Response of net ecosystem gas exchange to a simulated precipitation pulse in a semi-arid grassland: the role of native versus non-native grasses and soil texture. Oecologia 141:295–305
Jackson RB, Canadell J, Ehleringer JR et al (1996) A global analysis of root distributions for terrestrial biomes. Oecologia 108:389–411 doi:10.1007/BF00333714
Jackson RB, Schenk HJ, Jobbagy EG et al (2000) Belowground consequences of vegetation change and their treatment in models. Ecol Appl 10:470–483 doi:10.1890/1051-0761(2000)010[0470:BCOVCA]2.0.CO;2
Jackson RB, Banner JL, Jobbagy EG et al (2002) Ecosystem carbon loss with woody plant invasion of grasslands. Nature 418:623–626 doi:10.1038/nature00910
Kirschbaum MUF (1995) The temperature-dependence of soil organic-matter decomposition, and the effect of global warming on soil organic-C storage. Soil Biol Biochem 27:753–760 doi:10.1016/0038-0717(94)00242-S
Lin Q, Brookes PC (1999) Comparison of substrate induced respiration, selective inhibition and biovolume measurements of microbial biomass and its community structure in unamended, ryegrass-amended, fumigated and pesticide-treated soils. Soil Biol Biochem 31:1999–2014 doi:10.1016/S0038-0717(99)00122-4
Lunn DJ, Thomas A, Best N et al (2000) WinBUGS-A Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 10:325–337 doi:10.1023/A:1008929526011
NCDC (2008) Available at http://www.ncdc.noaa.gov/oa/ncdc.html. National Oceanic and Atmospheric Association, Asheville, NC
Ogle K (2008) Hierarchical Bayesian statistics: merging experimental and modeling approaches in ecology. Ecol Appl (in press)
Ogle K, Barber JJ (2008) Bayesian data-model integration in plant physiological and ecosystem ecology. Prog Bot 69:281–311 doi:10.1007/978-3-540-72954-9_12
Paul EA, Harris D, Collins HP et al (1999) Evolution of CO2 and soil carbon dynamics in biologically managed, row-crop agroecosystems. Appl Soil Ecol 11:53–65 doi:10.1016/S0929-1393(98)00130-9
Polley HW, Johnson HB, Tischler CR (2003) Woody invasion of grasslands: evidence that CO2 enrichment indirectly promotes establishment of Prosopis glandulosa. Plant Ecol 164:85–94 doi:10.1023/A:1021271226866
Potts DL, Huxman TE, Scott RL et al (2006) The sensitivity of ecosystem carbon exchange to seasonal precipitation and woody plant encroachment. Oecologia 150:453–463 doi:10.1007/s00442-006-0532-y
Raich JW, Potter CS (1995) Global patterns of carbon-dioxide emissions from soils. Glob Biogeochem Cycles 9:23–36 doi:10.1029/94GB02723
Raich JW, Schlesinger WH (1992) The global carbon-dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus Ser B Chem Phys Meteorol 44:81–99 doi:10.1034/j.1600-0889.1992.t01-1-00001.x
Ritz K, Dighton J, Giller KE et al (1994) Beyond the biomass. Wiley, Chichester, p 275
Robertson GP, Klingensmith KM, Klug MJ et al (1997) Soil resources, microbial activity, and primary production across an agricultural ecosystem. Ecol Appl 7:158–170 doi:10.1890/1051-0761(1997)007[0158:SRMAAP]2.0.CO;2
Saetre P, Stark JM (2005) Microbial dynamics and carbon and nitrogen cycling following re-wetting of soils beneath two semi-arid plant species. Oecologia 142:247–260 doi:10.1007/s00442-004-1718-9
Saleska SR, Harte J, Torn MS (1999) The effect of experimental ecosystem warming on CO2 fluxes in a montane meadow. Glob Chang Biol 5:125–141 doi:10.1046/j.1365-2486.1999.00216.x
Schimel DS, Braswell BH, Holland EA et al (1994) Climatic, edaphic, and biotic controls over storage and turnover of carbon in soils. Glob Biogeochem Cycles 8:279–293 doi:10.1029/94GB00993
Schipper LA, Degens BP, Sparling GP et al (2001) Changes in microbial heterotrophic diversity along five plant successional sequences. Soil Biol Biochem 33:2093–2103 doi:10.1016/S0038-0717(01)00142-0
Schuur EAG, Trumbore SE (2006) Partitioning sources of soil respiration in boreal black spruce forest using radiocarbon. Glob Chang Biol 12:165–176 doi:10.1111/j.1365-2486.2005.01066.x
Scott RL, Shuttleworth WJ, Goodrich DC et al (2000) The water use of two dominant vegetation communities in a semiarid riparian ecosystem. Agric For Meteorol 105:241–256 doi:10.1016/S0168-1923(00)00181-7
Sherrod LA, Dunn G, Peterson GA et al (2002) Inorganic carbon analysis by modified pressure-calcimeter method. Soil Sci Soc Am J 66:299–305
Thirukkumaran CM, Parkinson D (2000) Microbial respiration, biomass, metabolic quotient and litter decomposition in a lodgepole pine forest floor amended with nitrogen and phosphorous fertilizers. Soil Biol Biochem 32:59–66 doi:10.1016/S0038-0717(99)00129-7
Titlyanova AA, Romanova IP, Kosykh NP et al (1999) Pattern and process in above-ground and below-ground components of grassland ecosystems. J Veg Sci 10:307–320 doi:10.2307/3237060
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19:703–707 doi:10.1016/0038-0717(87)90052-6
Waldrop MP, Firestone MK (2006) Response of microbial community composition and function to soil climate change. Microb Ecol 52:716–724 doi:10.1007/s00248-006-9103-3
Wardle DA (2002) Communities and ecosystems: linking the aboveground and belowground components. Princeton University Press, Princeton, p 404
West AW, Sparling GP (1986) Modifications to the substrate-induced respiration method to permit measurement of microbial biomass in soils of differing water contents. J Microbiol Methods 5:177–189 doi:10.1016/0167-7012(86)90012-6
Wikle CK (2003) Hierarchical models in environmental science. Int Stat Rev 71:181–199
Williams MW, Brooks PD, Seastedt T (1998) Nitrogen and carbon soil dynamics in response to climate change in a high-elevation ecosystem in the Rocky Mountains, USA. Arct Alp Res 30:26–30 doi:10.2307/1551742
Wullschleger SD, Lynch JP, Berntson GM (1994) Modeling the belowground response of plants and soil biota to edaphic and climatic-change—what can we expect to gain. Plant Soil 165:149–160 doi:10.1007/BF00009971
Zak DR, Kling GW (2006) Microbial community composition and function across an arctic tundra landscape. Ecology 87:1659–1670 doi:10.1890/0012-9658(2006)87[1659:MCCAFA]2.0.CO;2
Acknowledgements
We thank Dr. David Williams and Dr. Russell Scott for the access to field sites and intellectual contributions; Greg Barron-Gafford, Ben Collins, Kevin “the Red” Gilliam, and Amelia Hazard for the field assistance; and Mary Kay Amistadi and Jon Chorover, School of Natural Resources, University of Arizona for the TOC analysis of microbial biomass samples. We acknowledge funding from SAHRA (Sustainability of Semi-Arid Hydrology and Riparian Areas) under the STC program of NSF, and NSF awards to TEH, Jake F. Weltzin, and David G. Williams. The experiments herein comply with the current laws of the USA. The statistical analysis was partly supported by a DOE NICCR grant (K.O., T.H.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Klaus Butterbach-Bahl.
Rights and permissions
About this article
Cite this article
Cable, J.M., Ogle, K., Tyler, A.P. et al. Woody plant encroachment impacts on soil carbon and microbial processes: results from a hierarchical Bayesian analysis of soil incubation data. Plant Soil 320, 153–167 (2009). https://doi.org/10.1007/s11104-008-9880-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9880-1