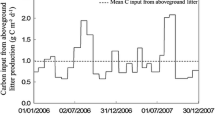
Abstract
Current understanding of carbon cycling in terrestrial ecosystem views “quantity” of litter input as a parameter determining the size of soil C pools and soil respired CO2: quantity of litter input is not considered a driving factor affecting the patterns of terrestrial ecosystem processes. Emma J. Sayer and collaborators demonstrated that this may not be the case! With a neat and elegant, for its simplicity, manipulation experiment, Sayer et al. (this issue) showed how increasing input of leaf litter affects the patterns of root distribution along the soil profile, in a relatively nutrient rich tropical soil. In their study, roots responded rapidly to changes in fresh leaf litter input and appeared to closely follow the patterns of litter decomposition. Until this study, root exploitation of standing litter was seen solely as an adaptation to nutrient shortage in the mineral soil. Feedback processes between leaf litterfall, decomposition and root dynamics are envisaged. With global climate change likely to alter plant productivity and litterfall, similar feedbacks, if confirmed, will need to be included in terrestrial ecosystem C modelling.
Similar content being viewed by others
References
B Berg (1987) ArticleTitleDynamics of nitrogen (15N) in decomposing Scots pine (Pinus sylvestris) needle litter. Long-term decomposition in a Scots pine forest. VI Can. J. Bot. 66 1539–1546
M F Cotrufo P Ineson A Scott (1998) ArticleTitleElevated CO2 reduces the nitrogen concentration of plant tissues Glob. Change Biol. 4 43–54 Occurrence Handle10.1046/j.1365-2486.1998.00101.x
E Cuevas E Medina (1988) ArticleTitleNutrient dynamics within Amazonian forests.2. Fine root-growth, nutrient availability and leaf litter decomposition Oecologia 76 222–235 Occurrence Handle10.1007/BF00379956
J F Dormaar (1990) ArticleTitleEffect of active roots on the decomposition of soil organic materials Biol. Fertil. Soils 10 121–126 Occurrence Handle1:CAS:528:DyaK3MXkvFWisLk%3D
R Fujimaki T P McGonigle H Takeda (2004) ArticleTitleSoil micro-habitat effects on fine roots of Chamaecyparis obtusa Endl.: A field experiment using root ingrowth cores Plant Soil 266 325–332 Occurrence Handle10.1007/s11104-005-2282-8 Occurrence Handle1:CAS:528:DC%2BD2MXpsFGitQ%3D%3D
R L Gadgil P D Gadgil (1971) ArticleTitleMycorrhiza and litter decomposition Nature 233 133 Occurrence Handle10.1038/233133a0 Occurrence Handle1:STN:280:DC%2BD2Mvgt1Wrtw%3D%3D Occurrence Handle16063238
M R Hoosbeek M Lukac D Dam Particlevan D L Godbold E J Velthorst F A Biondi A Peressotti M F Cotrufo P Angelis ParticleDe G Scarascia-Mugnozza (2004) ArticleTitleMore new carbon in the mineral soil of a poplar plantation under free air carbon enrichment (FACE): Cause of increased priming effect? Glob. Biogeochem. Cycle 18 IssueID1040 1041–1047
J D Joslin G S Henderson (1987) ArticleTitleOrganic matter and nutrients associated with fine root turnover in a white oak stand Forest Sci. 33 330–346
Y Kuzyakov (2002) ArticleTitleReview: Factors affecting rhizosphere priming effects J. Plant Nutr. Soil Sci. 165 382–396 Occurrence Handle10.1002/1522-2624(200208)165:4<382::AID-JPLN382>3.0.CO;2-# Occurrence Handle1:CAS:528:DC%2BD38XmsVKgtb8%3D
J P Laclau F Toutain A T M’Bou M Arnaud R Joffre J Ranger (2004) ArticleTitleThe function of the superficial root mat in the biogeochemical cycles of nutrients in Congolese Eucalyptus plantations Ann. Bot. 93 249–261 Occurrence Handle10.1093/aob/mch035 Occurrence Handle1:CAS:528:DC%2BD2cXjtVCksrw%3D Occurrence Handle14749252
E Personeni P Loiseau (2004) ArticleTitleHow does the nature of living and dead roots affect the residence time of carbon in the root litter continuum? Plant Soil 267 129–141 Occurrence Handle10.1007/s11104-005-4656-3 Occurrence Handle1:CAS:528:DC%2BD2MXht1Wktbk%3D
EJ Sayer EVJ Tanner AW Cheesman (2006 ) ArticleTitleIncreased litterfall changes fine root distribution in a moist tropical forest Plant Soil 281 5–13
W H Schlesinger J Lichter (2001) ArticleTitleLimited carbon storage in soil and litter of experimental forest plots under increased atmospheric CO2 Nature 411 466–469 Occurrence Handle10.1038/35078060 Occurrence Handle1:CAS:528:DC%2BD3MXkt1aqu74%3D Occurrence Handle11373676
J A Subke V Hahn G Battipaglia S Linder N Buchmann M F Cotrufo (2004) ArticleTitleFeedback interactions between needle litter decomposition and rhizosphere activity Oecologia 139 551–559 Occurrence Handle10.1007/s00442-004-1540-4 Occurrence Handle15042460
K A Vogt D J Vogt P A Palmiotto P Boon J O’Hara H Asbjornsen (1996) ArticleTitleReview of root dynamics in forest ecosystems grouped by climate, climatic forest type and species Plant Soil 187 159–219 Occurrence Handle10.1007/BF00017088 Occurrence Handle1:CAS:528:DyaK2sXjt12qsLg%3D
S D Wullschleger R J Norby C A Gunderson (1997) Forest trees and their response to atmospheric CO2 enrichment. A compilation of results L H J Allen M B Kirkham D M Olszyk C E Whitman (Eds) Advances in Carbon Dioxide Effects Research American Society of Agronomy Special Publication Madison, WI 79–100
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cotrufo, M.F. Quantity of Standing Litter: A Driving Factor of Root Dynamics. Plant Soil 281, 1–3 (2006). https://doi.org/10.1007/s11104-005-3506-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11104-005-3506-7