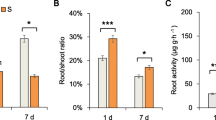
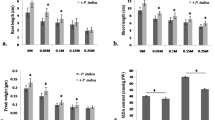
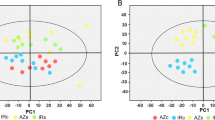
Abstract
The root endophytic fungus Piriformospora indica enhances plant adaptation to environmental stress based on general and non-specific plant species mechanisms. In the present study, we integrated the ionomics, metabolomics, and transcriptomics data to identify the genes and metabolic regulatory networks conferring salt tolerance in P. indica-colonized barley plants. To this end, leaf samples were harvested at control (0 mM NaCl) and severe salt stress (300 mM NaCl) in P. indica-colonized and non-inoculated barley plants 4 weeks after fungal inoculation. The metabolome analysis resulted in an identification of a signature containing 14 metabolites and ions conferring tolerance to salt stress. Gene expression analysis has led to the identification of 254 differentially expressed genes at 0 mM NaCl and 391 genes at 300 mM NaCl in P. indica-colonized compared to non-inoculated samples. The integration of metabolome and transcriptome analysis indicated that the major and minor carbohydrate metabolism, nitrogen metabolism, and ethylene biosynthesis pathway might play a role in systemic salt-tolerance in leaf tissue induced by the root-colonized fungus.








Similar content being viewed by others
References
Alikhani M, Khatabi B, Sepehri M, Nekouei MK, Mardi M, Salekdeh GH (2013) A proteomics approach to study the molecular basis of enhanced salt tolerance in barley (Hordeum vulgare L.) conferred by the root mutualistic fungus Piriformospora indica. Mol BioSyst 9:1498–1510
Baltruschat H, Fodor J, Harrach BD, Niemczyk E, Barna B, Gullner G, Janeczko A, Kogel KH, Schafer P, Schwarczinger I, Zuccaro A, Skoczowski A (2008) Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol 180:501–510
Barkla BJ, Castellanos‐Cervantes T, Diaz de León JL, Matros A, Mock H-P, Perez‐Alfocea F, Salekdeh GH, Witzel K, Zörb C (2013) Elucidation of salt stress defense and tolerance mechanisms of crop plants using proteomics—current achievements and perspectives. Proteomics 13:1885–1900
Barazani O, von Dahl CC, Baldwin IT (2007) Sebacina vermifera promotes the growth and fitness of Nicotiana attenuata by inhibiting ethylene signaling. Plant Physiol 144:1223–1232
Baud S, Bellec Y, Miquel M, Bellini C, Caboche M, Lepiniec L, Faure JD, Rochat C (2004) gurke and pasticcino3 mutants affected in embryo development are impaired in acetyl-CoA carboxylase. EMBO Rep 5:515–520
Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273:948–950
Bianchi G, Gamba A, Limiroli R, Pozzi N, Elster R, Salamini F, Bartels D (1993) The unusual sugar composition in leaves of the resurrection plant Myrothamnus flabellifolia. Physiol Plant 87:223–226
Blee KA, Anderson AJ (2002) Transcripts for genes encoding soluble acid invertase and sucrose synthase accumulate in root tip and cortical cells containing mycorrhizal arbuscules. Plant Mol Biol 50:197–211
Bolstad BM, Irizarry RA, Åstrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193
Borsani O, Zhu J, Verslues PE, Sunkar R, Zhu J-K (2005) Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulate salt tolerance in Arabidopsis. Cell 123:1279–1291
Bouché N, Lacombe B, Fromm H (2003) GABA signaling: a conserved and ubiquitous mechanism. Trends Cell Biol 13:607–610
Chinnusamy V, Zhu J-K (2009) Epigenetic regulation of stress responses in plants. Curr Opin Plant Biol 12:133–139
Choi C-S, Sano H (2007) Abiotic-stress induces demethylation and transcriptional activation of a gene encoding a glycerophosphodiesterase-like protein in tobacco plants. Mol Genet Genom 277:589–600
Drennan P, Smith M, Goldsworthy D, Van Staden J (1993) The occurrence of trehalose in the leaves of the desiccation-tolerant angiosperm Myrothamnus flabellifolius welw. J Plant Physiol 142:493–496
Fahad S, Hussain S, Matloob A, Khan FA, Khaliq A, Saud S, Hassan S, Shan D, Khan F, Ullah N (2014) Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul 75:391–404
Fedorov DN, Ekimova GA, Doronina NV, Trotsenko YA (2013) 1-Aminocyclopropane-1-carboxylate (ACC) deaminases from Methylobacterium radiotolerans and Methylobacterium nodulans with higher specificity for ACC. FEMS Microbiol Lett 343:70–76
Feng Y, Yin Y, Fei S (2015) Down-regulation of BdBRI1, a putative brassinosteroid receptor gene produces a dwarf phenotype with enhanced drought tolerance in Brachypodium distachyon. Plant Sci 234:163–173
Friedrichsen DM, Nemhauser J, Muramitsu T, Maloof JN, Alonso J, Ecker JR, Furuya M, Chory J (2002) Three redundant brassinosteroid early response genes encode putative bHLH transcription factors required for normal growth. Genetics 162:1445–1456
Ghabooli M, Khatabi B, Ahmadi FS, Sepehri M, Mirzaei M, Amirkhani A, Jorrin-Novo JV, Salekdeh GH (2013) Proteomics study reveals the molecular mechanisms underlying water stress tolerance induced by Piriformospora indica in barley. J Proteom 94:289–301
Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. New perspectives and approaches in plant growth-promoting Rhizobacteria research. Springer, New York, pp 329–339
Gordon AJ, Minchin FR, James CL, Komina O (1999) Sucrose synthase in legume nodules is essential for nitrogen fixation. Plant Physiol 120:867–878
Gruber BD, Giehl RF, Friedel S, von Wirén N (2013) Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol 163:161–179
Heinzel N, Rolletschek H (2011) Primary metabolite analysis of plant material using a triple quadrupole MS coupled to a monolith anion-exchange column. Dionex, Customer application note
Hoshida H, Tanaka Y, Hibino T, Hayashi Y, Tanaka A, Takabe T, Takabe T (2000) Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase. Plant Mol Biol 43:103–111
Huong PG, Kumari R, Singh A, Malla R, Prasad R, Sachdev M, Kaldorf M, Buscot F, Oelmüller R, Hampp R (2004) Axenic culture of symbiotic fungus Piriformospora indica. In: Plant surface microbiology. Springer, Germany, pp 593–613
Jouyban Z (2012) The effects of salt stress on plant growth. Tech J Eng Appl Sci 2:7–10
Kartal G, Temel A, Arican E, Gozukirmizi N (2009) Effects of brassinosteroids on barley root growth, antioxidant system and cell division. Plant Growth Regul 58:261–267
Khan AL, Hamayun M, Kang S-M, Kim Y-H, Jung H-Y, Lee J-H, Lee I-J (2012) Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: an example of Paecilomyces formosus LHL10. BMC Microbiol 12:3
Khatabi B, Molitor A, Lindermayr C, Pfiffi S, Durner J, von Wettstein D, Kogel KH, Schäfer P (2012) Ethylene supports colonization of plant roots by the mutualistic fungus Piriformospora indica. PLoS ONE 7:e35502
Kim J-M, Sasaki T, Ueda M, Sako K, Seki M (2015) Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front Plant Sci 6:114
Knight H, Trewavas AJ, Knight MR (1997) Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J 12:1067–1078
Koch AM, Kuhn G, Fontanillas P, Fumagalli L, Goudet J, Sanders IR (2004) High genetic variability and low local diversity in a population of arbuscular mycorrhizal fungi. Proc Natl Acad Sci USA 101:2369–2374
Koornneef M, Jorna M, Brinkhorst-Van der Swan D, Karssen C (1982) The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) Heynh. Theor Appl Genet 61:385–393
Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63:1593–1608
Krishna P (2003) Brassinosteroid-mediated stress responses. J Plant Growth Regul 22:289–297
Lahrmann U, Ding Y, Banhara A, Rath M, Hajirezaei MR, Döhlemann S, von Wirén N, Parniske M, Zuccaro A (2013) Host-related metabolic cues affect colonization strategies of a root endophyte. Proc Natl Acad Sci 110:13965–13970
Laparre J, Malbreil M, Letisse F, Portais JC, Roux C, Bécard G, Puech-Pagès V (2014) Combining metabolomics and gene expression analysis reveals that propionyl-and butyryl-carnitines are involved in late stages of arbuscular mycorrhizal symbiosis. Mol Plant 7:554–566
Läuchli A, Grattan S (2007) Plant growth and development under salinity stress. Advances in molecular breeding toward drought and salt tolerant crops. Springer, New York, pp 1–32
Maere S, Heymans K, Kuiper M (2005) BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21:3448–3449
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444:139–158
Mansour MMF (1998) Protection of plasma membrane of onion epidermal cells by glycinebetaine and proline against NaCl stress. Plant Physiol Biochem 36:767–772
Maraña C, García-Olmedo F, Carbonero P (1990) Differential expression of two types of sucrose synthase-encoding genes in wheat in response to anaerobiosis, cold shock and light. Gene 88:167–172
Matzke MA, Mosher RA (2014) RNA-directed DNA methylation: an epigenetic pathway of increasing complexity. Nat Rev Genet 15:394–408
Mayer M, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62:670–684
Mirouze M, Paszkowski J (2011) Epigenetic contribution to stress adaptation in plants. Curr Opin Plant Biol 14:267–274
Morán-Diez E, Rubio B, Domínguez S, Hermosa R, Monte E, Nicolás C (2012) Transcriptomic response of Arabidopsis thaliana after 24 h incubation with the biocontrol fungus Trichoderma harzianum. J Plant Physiol 169:614–620
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta (BBA)-Bioenerg 1767:414–421
Noguchi T, Fujioka S, Choe S, Takatsuto S, Yoshida S, Yuan H, Feldmann KA, Tax FE (1999) Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol 121:743–752
Pattanagul W, Thitisaksakul M (2008) Effect of salinity stress on growth and carbohydrate metabolism in three rice (Oryza sativa L.) cultivars differing in salinity tolerance. IJEB 46:736–742
Paul MJ, Primavesi LF, Jhurreea D, Zhang Y (2008) Trehalose metabolism and signaling. Annu Rev Plant Biol 59:417–441
Qiang X, Weiss M, Kogel KH, Schafer P (2011) Piriformospora indica: a mutualistic basidiomycete with an exceptionally large plant host range. Mol Plant Pathol 13(5):508–518
Rai V (2002) Role of amino acids in plant responses to stresses. Biol Plant 45:481–487
Rashmi K, Latha JNL, Sowjanya TN, Kiranmayi P, Rao MV, Menon C, Mohan PM (2003) Colonization of cruciferous plants by Piriformospora indica. Curr Sci 85:1672
Rodrigues CRF, Silva EN, Ferreira-Silva SL, Voigt EL, Viégas RA, Silveira JAG (2013) High K+ supply avoids Na+ toxicity and improves photosynthesis by allowing favorable K+: Na+ ratios through the inhibition of Na+ uptake and transport to the shoots of Jatropha curcas plants. J Plant Nutr Soil Sci 176:157–164
Sahay N, Varma A (1999) Piriformospora indica: a new biological hardening tool for micropropagated plants. FEMS Microbiol Lett 181:297–302
Sana TR, Fischer S, Wohlgemuth G, Katrekar A, K-h Jung, Ronald PC, Fiehn O (2010) Metabolomic and transcriptomic analysis of the rice response to the bacterial blight pathogen Xanthomonas oryzae pv. oryzae. Metabolomics 6:451–465
Schliemann W, Ammer C, Strack D (2008) Metabolite profiling of mycorrhizal roots of Medicago truncatula. Phytochemistry 69:112–146
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
Scott B, Takemoto D, Tanaka A (2007) Fungal endophyte production of reactive oxygen species is critical for maintaining the mutualistic symbiotic interaction between Epichloë festucae and perennial ryegrass. Plant Signal Behav 2:171–173
Sherameti I, Shahollari B, Venus Y, Altschmied L, Varma A, Oelmüller R (2005) The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. J Biol Chem 280:26241–26247
Sirrenberg A, Göbel C, Grond S, Czempinski N, Ratzinger A, Karlovsky P, Santos P, Feussner I, Pawlowski K (2007) Piriformospora indica affects plant growth by auxin production. Physiol Plant 131:581–589
Sreenivasulu N, Radchuk V, Strickert M, Miersch O, Weschke W, Wobus U (2006) Gene expression patterns reveal tissue-specific signaling networks controlling programmed cell death and ABA-regulated maturation in developing barley seeds. Plant J 47:310–327
Usadel B, Poree F, Nagel A, Lohse M, Czedik-Eysenberg A, Stitt M (2009) A guide to using MapMan to visualize and compare Omics data in plants: a case study in the crop species, Maize. Plant Cell Environ 32:1211–1229
Vadassery J, Ritter C, Venus Y, Camehl I, Varma A, Shahollari B, Novák O, Strnad M, Ludwig-Müller J, Oelmüller R (2008) The role of auxins and cytokinins in the mutualistic interaction between Arabidopsis and Piriformospora indica. Mol Plant Microbe Interact 21:1371–1383
Vadassery J, Ranf S, Drzewiecki C, Mithöfer A, Mazars C, Scheel D, Lee J, Oelmüller R (2009) A cell wall extract from the endophytic fungus Piriformospora indica promotes growth of Arabidopsis seedlings and induces intracellular calcium elevation in roots. Plant J 59:193–206
Varma A, Verma S, Sahay N, Bütehorn B, Franken P (1999) Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl Environ Microbiol 65:2741–2744
Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T, Hückelhoven R, Neumann C, Von Wettstein D (2005) The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci USA 102:13386
Waller F, Mukherjee K, Deshmukh SD, Achatz B, Sharma M, Schäfer P, Kogel KH (2008) Systemic and local modulation of plant responses by Piriformospora indica and related Sebacinales species. J Plant Physiol 165:60–70
Wang H, Yang C, Zhang C, Wang N, Lu D, Wang J, Zhang S, Wang ZX, Ma H, Wang X (2011) Dual role of BKI1 and 14-3-3 s in brassinosteroid signaling to link receptor with transcription factors. Dev Cell 21:825–834
Wang X, Chang L, Wang B, Wang D, Li P, Wang L, Yi X, Huang Q, Peng M, Guo A (2013) Comparative proteomics of Thellungiella halophila leaves from plants subjected to salinity reveals the importance of chloroplastic starch and soluble sugars in halophyte salt tolerance. Mol Cell Proteom 12:2174–2195
Xia J, Sinelnikov IV, Han B, Wishart DS (2015) MetaboAnalyst 3.0: making metabolomics more meaningful. Nucleic Acids Res 43:W251–W257
Zhang J, Jia W, Yang J, Ismail AM (2006) Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res 97:111–119
Zhang Z, Zhang H, Quan R, Wang X-C, Huang R (2009) Transcriptional regulation of the ethylene response factor LeERF2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiol 150:365–377
Zhu J-K (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247
Zurbriggen MD, Carrillo N, Tognetti VB, Melzer M, Peisker M, Hause B, Hajirezaei MR (2009) Chloroplast-generated reactive oxygen species play a major role in localized cell death during the non-host interaction between tobacco and Xanthomonas campestris pv. vesicatoria. Plant J 60:962–973
Acknowledgments
We would like to thank Melanie Ruff at IPK-Gatersleben for the excellent technical assistance.
Author contributions
MG prepared the plant samples; MRG, MRH and MG performed metabolome analysis; PS performed macroarray analysis; MRG and BK analyzed the data; GHS designed the experiment; MRG, BK and GHS wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
Difference in plant dry weight between Piriformospora indica root-colonized and non-inoculated barley plants four weeks post inoculation in 0 mM NaCl (green color) and 300 mM NaCl (red color) conditions. Bars indicate standard error values for each treatment (n = 5). (TIFF 3686 kb)
Rights and permissions
About this article
Cite this article
Ghaffari, M.R., Ghabooli, M., Khatabi, B. et al. Metabolic and transcriptional response of central metabolism affected by root endophytic fungus Piriformospora indica under salinity in barley. Plant Mol Biol 90, 699–717 (2016). https://doi.org/10.1007/s11103-016-0461-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-016-0461-z