Abstract
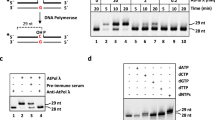
The initiation of DNA replication starts from origins and is controlled by a multiprotein complex, which involves about twenty protein factors. One of the important factors is hetrohexameric minichromosome maintenance (MCM2-7) protein complex which is evolutionary conserved and functions as essential replicative helicase for DNA replication. Here we report the isolation and characterization of a single subunit of pea MCM protein complex, the MCM6. The deduced amino acid (827) sequence contains all the known canonical MCM motifs including zinc finger, MCM specific Walker A and Walker B and arginine finger. The purified recombinant protein contains ATP-dependent 3′–5′ DNA helicase, ATP-binding and ATPase activities. The helicase activity was stimulated by replication fork like substrate and anti-MCM6 antibodies curtail all the enzyme activities of MCM6 protein. In vitro it self-interacts and forms a homohexamer which is active for DNA helicase and ATPase activities. The complete protein is required for self-interaction as the truncated MCM6 proteins were unable to self-interact. Western blot analysis and in vivo immunostaining followed by confocal microscopy showed the localization of MCM6 both in the nucleus and cytosol. These findings provide first direct evidence that single subunit MCM6 contains DNA helicase activity which is unique to plant MCM6 protein, as this activity was only reported for heteromultimers of MCM proteins in animal system. This discovery should make an important contribution to a better understanding of DNA replication in plants.






Similar content being viewed by others
References
Barry ER, Lovett JE, Costa A, Lea SM, Bell SD (2009) Intersubunit allosteric communication mediated by a conserved loop in the MCM helicase. Proc Natl Acad Sci USA 106:1051–1056
Bell SP, Dutta A (2002) DNA replication in eukaryotic cells. Annu Rev Biochem 71:333–374
Bochman ML, Schwacha A (2008) The Mcm2–7 complex has in vitro helicase activity. Mol Cell 31:287–293
Castellano MM, Boniotti MB, Caro E, Schnittger A, Gutierrez C (2004) DNA replication licensing affects cell proliferation or endoreplication in a cell type-specific manner. Plant Cell 16:2380–2393
Dambrauskas G, Aves SJ, Bryant JA, Francis D, Rogers J (2003) Genes encoding two essential DNA replication activation proteins, Cdc6 and Mcm3, exhibit very different patterns of expression in tobacco BY-2 cell cycle. J Exp Bot 54:699–706
Dresselhaus T, Srilunchang KO, Leljak-Levanic D, Schreiber DN, Garg P (2006) The fertilization-induced DNA replication factor MCM6 of maize shuttles between cytoplasm and nucleus, and is essential for plant growth and development. Plant Physiol 140:512–527
Forsburg SL (2004) Eukaryotic MCM proteins: beyond replication initiation. Microbiol Mol Biol Rev 68:109–131
Gozuacik D, Chami M, Lagorce D et al (2003) Identification and functional characterization of a new member of the human Mcm protein family: hMcm8. Nucleic Acids Res 31:570–579
Hyrien O, Marheineke K, Goldar A (2003) Paradoxes of eukaryotic DNA replication: MCM proteins and the random completion problem. Bioessays 25:116–125
Ishimi Y (1997) A DNA helicase activity is associated with an MCM4, -6, and -7 protein complex. J Biol Chem 272:24508–24513
Kanter DM, Bruck I, Kaplan DL (2008) Mcm subunits can assemble into two different active unwinding complexes. J Biol Chem 283:31172–31182
Kelman Z, Hurwitz J (2003) Structural lessons in DNA replication from the third domain of life. Nat Struct Biol 10:148–150
Kelman Z, Lee JK, Hurwitz J (1999) The single minichromosome maintenance protein of Methanobacterium thermoautotrophicum DeltaH contains DNA helicase activity. Proc Natl Acad Sci USA 96:14783–14788
Labib K, Kearsey SE, Diffley JF (2001) MCM2–7 proteins are essential components of prereplicative complexes that accumulate cooperatively in the nucleus during G1-phase and are required to establish, but not maintain, the S-phase checkpoint. Mol Biol Cell 12:3658–3667
Lee JK, Hurwitz J (2001) Processive DNA helicase activity of the minichromosome maintenance proteins 4, 6, and 7 complex requires forked DNA structures. Proc Natl Acad Sci USA 98:54–59
Lin DI, Aggarwal P, Diehl JA (2008) Phosphorylation of MCM3 on Ser-112 regulates its incorporation into the MCM2–7 complex. Proc Natl Acad Sci USA 105:8079–8084
Liu W, Pucci B, Rossi M, Pisani FM, Ladenstein R (2008) Structural analysis of the Sulfolobus solfataricus MCM protein N-terminal domain. Nucleic Acids Res 36:3235–3243
Liu Y, Richards TA, Aves SJ (2009) Ancient diversification of eukaryotic MCM DNA replication proteins. BMC Evol Biol 9:60
Maiorano D, Lemaitre JM, Mechali M (2000) Stepwise regulated chromatin assembly of MCM2–7 proteins. J Biol Chem 275:8426–8431
Misra S, Wu Y, Venkataraman G, Sopory SK, Tuteja N (2007) Heterotrimeric G-protein complex and G-protein-coupled receptor from a legume (Pisum sativum): role in salinity and heat stress and cross-talk with phospholipase C. Plant J 51:656–669
Ni DA, Sozzani R, Blanchet S et al (2009) The Arabidopsis MCM2 gene is essential to embryo development and its over-expression alters root meristem function. New Phytol 184:311–322
Pape T, Meka H, Chen S et al (2003) Hexameric ring structure of the full-length archaeal MCM protein complex. EMBO Rep 4:1079–1083
Patel SS, Picha KM (2000) Structure and function of hexameric helicases. Annu Rev Biochem 69:651–697
Sabelli PA, Burgess SR, Kush AK, Young MR, Shewry PR (1996) cDNA cloning and characterization of a maize homologue of the MCM proteins required for the initiation of DNA replication. Mol Gen Genet 252:125–136
Shultz RW, Lee TJ, Allen GC, Thompson WF, Hanley-Bowdoin L (2009) Dynamic localization of the DNA replication proteins MCM5 and MCM7 in plants. Plant Physiol 150:658–669
Springer PS, McCombie WR, Sundaresan V, Martienssen RA (1995) Gene trap tagging of PROLIFERA, an essential MCM2–3-5-like gene in Arabidopsis. Science 268:877–880
Takahashi K, Yamada H, Yanagida M (1994) Fission yeast minichromosome loss mutants mis cause lethal aneuploidy and replication abnormality. Mol Biol Cell 5:1145–1158
Tuteja N, Rahman K, Tuteja R, Falaschi A (1993) Human DNA helicase V, a novel DNA unwinding enzyme from HeLa cells. Nucleic Acids Res 21:2323–2329
Tuteja N, Tuteja R, Ochem A et al (1994) Human DNA helicase II: a novel DNA unwinding enzyme identified as the Ku autoantigen. EMBO J 13:4991–5001
Tuteja N, Phan TN, Tewari KK (1996) Purification and characterization of a DNA helicase from pea chloroplast that translocates in the 3′-to-5′ direction. Eur J Biochem 238:54–63
Tuteja N, Beven AF, Shaw PJ, Tuteja R (2001) A pea homologue of human DNA helicase I is localized within the dense fibrillar component of the nucleolus and stimulated by phosphorylation with CK2 and cdc2 protein kinases. Plant J 25:9–17
Tye BK (1999) MCM proteins in DNA replication. Annu Rev Biochem 68:649–686
Vashisht AA, Pradhan A, Tuteja R, Tuteja N (2005) Cold- and salinity stress-induced bipolar pea DNA helicase 47 is involved in protein synthesis and stimulated by phosphorylation with protein kinase C. Plant J 44:76–87
Yabuta N, Kajimura N, Mayanagi K et al (2003) Mammalian Mcm2/4/6/7 complex forms a toroidal structure. Genes Cells 8:413–4121
You Z, Komamura Y, Ishimi Y (1999) Biochemical analysis of the intrinsic Mcm4-Mcm6-Mcm7 DNA helicase activity. Mol Cell Biol 19:8003–8015
Yu Z, Feng D, Liang C (2004) Pairwise interactions of the six human MCM protein subunits. J Mol Biol 340:1197–1206
Zou L, Stillman B (1998) Formation of a preinitiation complex by S-phase cyclin CDK-dependent loading of Cdc45p onto chromatin. Science 280:593–596
Acknowledgments
Studies on DNA replication and plant abiotic stress tolerance in NT’s laboratory are partially supported by Department of Biotechnology (DBT), Government of India. We thank Dr. Xuan Hoi Pham for his initial help in the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.



Rights and permissions
About this article
Cite this article
Tran, N.Q., Dang, H.Q., Tuteja, R. et al. A single subunit MCM6 from pea forms homohexamer and functions as DNA helicase. Plant Mol Biol 74, 327–336 (2010). https://doi.org/10.1007/s11103-010-9675-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-010-9675-7