Abstract
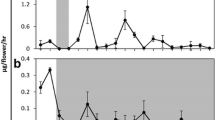
The white flowers of N. suaveolens emit a complex bouquet of fragrance volatiles. The dominant compounds are benzenoids (e.g. methyl benzoate, methyl salicylate, benzyl benzoate and benzyl salicylate), monoterpenes (1,8-cineole, limonene, sabinene, E-β-ocimene, β-β-myrcene, α- and β-pinene and α-terpineole) and sesquiterpenes (e.g. caryophyllene), which are all emitted at higher levels during the night. Here, we show that the simultaneous nocturnal emission of most monoterpenes is realized by a single floral-specific multi-product enzyme (1,8-cineole synthase, CIN), which synthesizes the monoterpenes of the “cineole cassette”. Interestingly, N. suaveolens is the only known taxon of the Suaveolentes section to have a flower emitting “cineole cassette of monoterpenes” which is otherwise typical for the Alatae section. Gene sequence analysis of CIN has revealed the highest similarities to other angiosperm monoterpene synthases from Vitis vinifera, Quercus ilex, Citrus unshiu and C. limon, which cluster in the same branch of the terpene synthase B subfamily. However, based on its synthesized products, N. suaveolens CIN shares similarity with enzymes of the Arabidopsis thaliana root and Salvia officinalis leaf. The N. suaveolens CIN gene is only expressed in the stigma/style tissue and petals. Thin sections of petals present the enzyme primarily in the adaxial and abaxial epidermis; this facilitates the comprehensive emission of volatiles in all spacial directions. The oscillation of monoterpene emission is a consequence of the regulation of the CIN gene by the circadian clock, with oscillations occurring at the level of transcript and protein accumulations and of enzyme activity. Light/dark or dark/light transition signals synchronize the slow-running endogenous clock. Two strategies for synchronized scent emission have been established in N. suaveolens flowers: (i) the synthesis of volatile organic compounds by a multi-product enzyme and (ii) the coordination of biosynthetic pathways by a circadian clock.









Similar content being viewed by others
Abbreviations
- LIS:
-
Linalool synthase
- CIN:
-
Cineole synthase
- GPP:
-
Geranyl pyrophosphate
- VOCs:
-
Volatile organic compounds
- LL:
-
Continuous illumination
- DD:
-
Continuous darkness
- LD :
-
Light/dark regime
- SPME:
-
Solid phase micro extraction
References
Altschul SF, Gish W, Miller W, Byers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:402–410
Andersson S (2006) Floral scent and butterfly pollinators. In: Dudareva N, Pichersky E (eds) Biology of floral scent. Taylor & Francis Group, Boca Raton, 199–217
Aoki S, Ito M (2000) Molecular phylogeny of Nicotiana (Solanaceae) based on the nucleotide sequence of the matK gene. Plant Biol 2:316–324
Asby M, Edwards PA (1990) Elucidation of the deficiency in two yeast coenzyme Q mutants. Characterization of the structural gene encoding hexaprenyl pyrophosphate synthetase. J Bio Chem 265:13157–13164
Aubourg S, Lecharny A, Bohlmann J (2002) Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol Gen Genomics 267:730–745
Ayasse M (2006) Floral scent and pollinator attraction in sexually deceptive orchids. In: Dudareva N, Pichersky E (eds) Biology of floral scent. Taylor & Francis Group, Boca Raton, 219–241
Bohlmann J, Meyer-Gauen G, Croteau RB (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA 95:4126–4133
Bohlmann J, Gershenzon J, Aubourg S (2000) Biochemical, molecular genetic and evolutionary aspects of defense-related terpenoid metabolism in conifers. Recent Adv Phytochem 34:109–150
Chang S, Puryear J, Caiurney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11:113–116
Chase MW, Knapp S, Cox AV, Clarkson JJ, Butsko Y, Joseph J, Savolainen V, Parokonny AS (2003) Molecular systematics, GISH and the origin of hybrid taxa in Nicotiana (Solanaceae). Ann Bot 92:107–127
Chen F, Tholl D, D’Auria JC, Farooq A, Pichersky E, Gershenzon J (2003) Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell 15:481–494
Chen F, Ro D-K, Petri J, Gershenzon J, Bohlmann J, Pichersky E, Tholl D (2004) Characterization of a root-specific Arabidopsis terpene synthase responsible for the formation of the volatile monoterpene 1,8-cineole. Plant Physiol 135:1956–1966
Cheng SH, Seemann JR (1998) Extraction and purification of RNA from plant tisue enriched in polysaccharides. Methods Mol Biol 68:27–32
Davis EM, Croteau R (2000) Cyclization enzymes in the biosynthesis of monoterpenes and diterpenes. Top Curr Chem 209:53–95
Dobson HEM (2006) Relationship between floral fragrance composition and type of pollinator. In: Dudareva N, Pichersky E (eds) Biology of floral scent. Taylor & Francis Group, Boca Raton, 147–197
Dudareva N, Cseke L, Blanc VM, Pichersky E (1996) Evolution of floral scent in Clarkia: novel patterns of S-linalool synthase gene expression in the C. breweri flower. Plant Cell 8:1137–1148
Dudareva N, Piechulla B, Pichersky E (2000) Biogeneses of floral scents. In: Janik J (eds) Horticultural reviews. vol 24. John Wiley & Sons, Inc, New York, 31–54
Dudareva N, Martin D, Kish CM, Kolosova N, Gorenstein N, Fäldt J (2003) (E)-β-ocimene and β-myrcene synthase genes of floral scent biosynthesis in snapdragon: function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell 15:1227–1241
Effmert U, Saschenbrecker S, Ross J, Negre F, Fraser CM, Noel JP, Dudareva N, Piechulla B (2005) Floral benzenoid carboxyl methyltransferases: from in vitro to in planta function. Phytochemistry 66:1211–1230
Effmert U, Buss D, Rohrbeck D, Piechulla B (2006) Localization of the synthesis and emission of scent compounds within the flower. In: Dudareva N, Pichersky E (eds) Biology of floral scent. New York, Tayler & Francis Group, Boca Raton, 105–123
Facchini PJ, Chappell J (1992) Gene familiy for an elicitor-induced sesquiterpene cyclase from tobacco. Proc Natl Acad Sci USA 89:11088–11092
Goodspeed TH (1954) The genus Nicotiana. Chronica Botanica Company, Walthman, Mass., USA
Gutermann I, Shalit M, Menda N, Piestun D, Dafny-Yellin M, Shalev G, Bar E, Davydov O, Ovadis M, Wang J, Adam Z, Pichersky E, Lewinsohn E, Zamir D, Vainstein A, Weiss D (2002) Rose scent: genomics approach to discovering novel floral fragrance-related genes. Plant Cell 14:2325–2338
Hoffrogge R, Mikschofski H, Piechulla B (2003) Suface plasmon resonance spectoscopy (SPR) interaction studies of the circadian-controlled tomato LHCa4*1 (CAB 11) protein with its promoter. Chronobiol Int 20:543–558
Knudsen JT, Gershenzon J (2006) The chemical diversity of floral scent. In: Dudareva N, Pichersky E (eds) Biology of floral scent. Taylor & Francis Group, Boca Raton, pp. 27–52
Knudsen JT, Eriksson R, Gershenzon J, Stahl B (2006) Diversity and distribution of floral scent. Bot Rev 72:1–120
Kolosova N, Gorenstein N, Kish CM, Dudareva N (2001a) Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. Plant Cell 13:2333–2347
Kolosova N, Shermann D, Karlson D, Dudareva N (2001b) Cellular and subcellular localization of S-adenosyl-L-methionine: benzoic acid carboxyl methyltransferase, the enzyme responsible for biosynthesis of the volatile ester methylbenzoate in snapdragon flowers. Plant Physiol 126:956–964
Loughrin JH, Hamilton-Kemp TR, Anderson RA, Hildebrand DF (1990) Volatiles from flowers of Nicotiana sylvestris, N. otophora and N. malus x domestica: headspace components and day/night changes in their relative concentrations. Phytochemistry 29:2473–2477
Loughrin JH, Hamilton-Kemp TR, Anderson RA, Hildebrand DF (1991) Circadian rhythm of volatile emission from flowers of Nicotiana sylvestris and N. suaveolens. Physiol Plant 83:492–496
Loughrin JH, Hamilton-Kemp TR, Burton HR, Anderson RA (1993) Effect of diurnal sampling on the headspace composition of detached Nicotiana suaveolens flowers. Phytochemistry 32:1417–1419
Lu S, Xu R, Jia JW, Pang J, Matsuda SPT, Chen XY (2002) Cloning and functional characterization of a β-pinene synthase from Artemisia annua that shows circadian pattern of expression. Plant Physiol 130:477–486
McClung CR (2006) Plant circadian rhythms. Plant Cell 18:792–803
Peters RJ, Croteau RB (2003) Alternative termination chemistries utilized by monoterpene cyclases: chimeric analysis of bornyl diphosphate, 1,8-cineole, and sabinene synthase. Arch Biochem Biophys 417:203–211
Pichersky E, Raguso RA, Lewinsohn E, Croteau R (1994) Floral scent production in Clarkia (Onagraceae). I. Localization and developmental modulation of monoterpene emission and linalool synthase activity. Plant Physiol 106:1533–1540
Pichersky E, Lewinsohn E, Croteau R (1995) Purification and characterization of S-linalool synthase, an enzyme involved in the production of floral scent in Clarkia breweri. Archiv Biochem Biophys 316:803–807
Pott MB, Pichersky E, Piechulla B (2002) Evening-specific oscillations of scent emission, SAMT enzyme activity, and SAMT mRNA in flowers of Stephanotis floribunda. J Plant Physiol 159:925–934
Pott MB, Effmert U, Piechulla B (2003) Transcriptional and post-translational regulation of S-adenosyl-L-methionine: salicylic acid carboxyl methyltransferase (SAMT) during Stephanotis floribunda flower development. J Plant Physiol 160:635–643
Pott BM, Hippauf F, Saschenbrecker S, Chen F, Ross J, Kiefer I, Slusarenko A, Noel JP, Pichersky E, Effmert U, Piechulla B (2004) Biochemical and structural characterization of benzenoid carboxyl methyltransferases involved in floral scent production in Stephanotis floribunda and Nicotiana suaveolens. Plant Physiol 135:1946–1955
Raguso RA, Levin R.A, Foose SE, Homberg MW, McDade LA (2003) Fragrance chemistry, nocturnal rhythms and pollination “syndromes” in Nicotiana. Phytochemistry 63:265–284
Raguso RA, Schlumpberger BO, Kaczorowski RL, Holtsford TP (2006) Phylogenetic fragrance patterns in Nicotiana sections Alatae and Suaveolentes. Phytochemistry 67:1931–1942
Rohrbeck D, Buss D, Effmert U, Piechulla B (2006) Localization of methyl benzoate synthesis and emission in Stephanotis florbunda and Nicotiana suaveolens flowers. Plant Biol 8:615–626
Schiestl FP, Ayasse M, Paulus HF, Erdmann D, Franke W (1997) Variation of floral scent emission and postpollination changes in individual flowers of Ophrys sphegodes subsp. shegodes. J Chem Ecol 23:2881–2895
Shimada T, Endo T, Fujii H, Hara M, Omura M (2005) Isolation and characterization of (E)-β-ocimene and 1,8-cineole synthases in Citrus unshiu Marc. Plant Sci 168:987–995
Tollsten L (1993) A multivariante approach to post-pollination changes in the floral scent of Platanthera bifolia (Orchidaceae). Nord J Bot 13:495–499
Trapp SC, Croteau RB (2001) Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 158:811–832
Turner GW, Gershenzon J, Croteau RB (2000a) Distributions of peltate glandular trichomes on developing leaves of peppermint. Plant Physiol 124:655–664
Turner GW, Gershenzon J, Croteau RB (2000b) Development of peltate glandular trichomes of peppermint. Plant Physiol 124:665–680
Wang J, Dudareva N, Bhakta S, Raguso RA, Pichersky E (1997) Floral scent production in Clarkia breweri (Onagraceae). II. Localization and developmental modulation of the enzyme S’adenosyl-L-methionine:(iso)eugenol O-methyltransferase and phenylpropanoid emission. Plant Physiol 114:213–221
Werker E, Ravid U, Putievsky E (1985) Glandular hairs and their secretions in the vegetative and reproductive organs of Salvia sclarea and S. dominica. Isr J Bot 34:239–252
Wise ML, Savage TJ, Katahira E, Croteau R (1998) Monoterpene synthases from common sage (Salvia officinales). J Biol Chem 273:14891–14899
Acknowledgements
The authors thank Rita Heese (University of Rostock) for her technical assistance, Diana Rohrbeck for her help with in situ hybridizations and Sandra Saschenbrecker for many RNA samples. We also thank Natalia Dudareva (University Purdue, Indiana, USA) and Eran Pichersky (University Ann Arbor, Michigan, USA) for their initial help in clone isolation and enzyme characterization. This project was financially supported by the DFG to BP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roeder, S., Hartmann, AM., Effmert, U. et al. Regulation of simultaneous synthesis of floral scent terpenoids by the 1,8-cineole synthase of Nicotiana suaveolens . Plant Mol Biol 65, 107–124 (2007). https://doi.org/10.1007/s11103-007-9202-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-007-9202-7