Abstract
Purpose
Functional pituitary adenomas (FPAs) cause severe neuro-endocrinopathies including Cushing’s disease (CD) and acromegaly. While many are effectively cured following FPA resection, some encounter disease recurrence/progression or hormonal non-remission requiring adjuvant treatment. Identification of risk factors for suboptimal postoperative outcomes may guide initiation of adjuvant multimodal therapies.
Methods
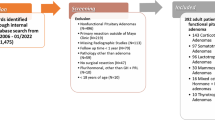
Patients undergoing endonasal transsphenoidal resection for CD, acromegaly, and mammosomatotroph adenomas between 1992 and 2019 were identified. Good outcomes were defined as hormonal remission without imaging/biochemical evidence of disease recurrence/progression, while suboptimal outcomes were defined as hormonal non-remission or MRI evidence of recurrence/progression despite adjuvant treatment. Multivariate regression modeling and multilayered neural networks (NN) were implemented. The training sets randomly sampled 60% of all FPA patients, and validation/testing sets were 20% samples each.
Results
348 patients with mean age of 41.7 years were identified. Eighty-one patients (23.3%) reported suboptimal outcomes. Variables predictive of suboptimal outcomes included: Requirement for additional surgery in patients who previously had surgery and continue to have functionally active tumor (p = 0.0069; OR = 1.51, 95%CI 1.12–2.04), Preoperative visual deficit not improved after surgery (p = 0.0033; OR = 1.12, 95%CI 1.04–1.20), Transient diabetes insipidus (p = 0.013; OR = 1.27, 95%CI 1.05–1.52), Higher MIB-1/Ki-67 labeling index (p = 0.038; OR = 1.08, 95%CI 1.01–1.15), and preoperative low cortisol axis (p = 0.040; OR = 2.72, 95%CI 1.06–7.01). The NN had overall accuracy of 87.1%, sensitivity of 89.5%, specificity of 76.9%, positive predictive value of 94.4%, and negative predictive value of 62.5%. NNs for all FPAs were more robust than for CD or acromegaly/mammosomatotroph alone.
Conclusion
We demonstrate capability of predicting suboptimal postoperative outcomes with high accuracy. NNs may aid in stratifying patients for risk of suboptimal outcomes, thereby guiding implementation of adjuvant treatment in high-risk patients.


Similar content being viewed by others
References
Ostrom QT, Cioffi G, Gittleman H et al (2019) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol 21:v1–v100
Melmed S (2011) Pathogenesis of pituitary tumors. Nat Rev Endocrinol 7:257–266
Melmed S (2020) Pituitary-tumor endocrinopathies. N Engl J Med 382:937–950
Aflorei ED, Korbonits M (2014) Epidemiology and etiopathogenesis of pituitary adenomas. J Neurooncol 117:379–394
Akin S, Isikay I, Soylemezoglu F et al (2016) Reasons and results of endoscopic surgery for prolactinomas: 142 surgical cases. Acta Neurochir 158:933–942
Kim JH, Hur KY, Lee JH et al (2017) Outcome of endoscopic transsphenoidal surgery for acromegaly. World Neurosurg 104:272–278
Mehta GU, Lonser RR (2017) Management of hormone-secreting pituitary adenomas. Neuro Oncol 19:762–773
Campbell PG, Kenning E, Andrews DW et al (2010) Outcomes after a purely endoscopic transsphenoidal resection of growth hormone-secreting pituitary adenomas. Neurosurg Focus 29:E5
Han Y-L, Chen D-M, Zhang C et al (2018) Retrospective analysis of 52 patients with prolactinomas following endoscopic endonasal transsphenoidal surgery. Medicine 97:e13198
Berker M, Hazer DB, Yücel T et al (2012) Complications of endoscopic surgery of the pituitary adenomas: analysis of 570 patients and review of the literature. Pituitary 15:288–300
Broersen LHA, Biermasz NR, van Furth WR et al (2018) Endoscopic vs. microscopic transsphenoidal surgery for Cushing’s disease: a systematic review and meta-analysis. Pituitary 21:524–534
Gondim JA, Schops M, de Almeida JPC et al (2010) Endoscopic endonasal transsphenoidal surgery: surgical results of 228 pituitary adenomas treated in a pituitary center. Pituitary 13:68–77
Paluzzi A, Fernandez-Miranda JC, Tonya Stefko S et al (2014) Endoscopic endonasal approach for pituitary adenomas: a series of 555 patients. Pituitary 17:307–319
Todeschini AB, Santos ARLD, Dolci RLL et al (2019) Long term follow-up after endoscopic endonasal approach for the treatment of cushing’s disease. J Neurol Surg B Skull Base 80:306–309
Wang F, Zhou T, Wei S et al (2015) Endoscopic endonasal transsphenoidal surgery of 1166 pituitary adenomas. Surg Endosc 29:1270–1280
Nayak P, Montaser AS, Hu J et al (2018) Predictors of postoperative diabetes insipidus following endoscopic resection of pituitary adenomas. J Endocr Soc 2:1010–1019
Babu H, Ortega A, Nuno M et al (2017) Long-term endocrine outcomes following endoscopic endonasal transsphenoidal surgery for acromegaly and associated prognostic factors. Neurosurgery 81:357–366
Hofstetter CP, Nanaszko MJ, Mubita LL et al (2012) Volumetric classification of pituitary macroadenomas predicts outcome and morbidity following endoscopic endonasal transsphenoidal surgery. Pituitary 15:450–463
Zhang K, Shen M, Qiao N et al (2020) Surgical outcomes and multidisciplinary management strategy of Cushing’s disease: a single-center experience in China. Neurosurg Focus 48:E7
Sarkar S, Rajaratnam S, Chacko G, et al (2016) Pure endoscopic transsphenoidal surgery for functional pituitary adenomas: outcomes with Cushing’s disease. Acta Neurochir 158:77–86; discussion 86
Lobatto DJ, de Vries F, Zamanipoor Najafabadi AH et al (2018) Preoperative risk factors for postoperative complications in endoscopic pituitary surgery: a systematic review. Pituitary 21:84–97
Nadezhdina EY, Rebrova OY, Grigoriev AY et al (2019) Prediction of recurrence and remission within 3 years in patients with Cushing disease after successful transnasal adenomectomy. Pituitary 22:574–580
Micko ASG, Wöhrer A, Höftberger R et al (2017) MGMT and MSH6 immunoexpression for functioning pituitary macroadenomas. Pituitary 20:643–653
Biller BMK, Grossman AB, Stewart PM et al (2008) Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93:2454–2462
Katznelson L, Laws ER Jr, Melmed S et al (2014) Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99:3933–3951
Melmed S, Casanueva FF, Hoffman AR et al (2011) Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:273–288
van Buuren S, Groothuis-Oudshoorn K (2011) mice: Multivariate Imputation by Chained Equations inR. J Statist Software 45
Staartjes VE, Serra C, Muscas G et al (2018) Utility of deep neural networks in predicting gross-total resection after transsphenoidal surgery for pituitary adenoma: a pilot study. Neurosurg Focus 45:E12
Serra C, Staartjes VE, Maldaner N et al (2018) Predicting extent of resection in transsphenoidal surgery for pituitary adenoma. Acta Neurochir 160:2255–2262
Hollon TC, Parikh A, Pandian B et al (2018) A machine learning approach to predict early outcomes after pituitary adenoma surgery. Neurosurg Focus 45:E8
Woods C, Thompson CJ (2008) Risk of diabetes insipidus after pituitary surgery. Expert Rev Endocrinol Metab 3:23–27
Carman N, Kay C, Petersen A et al (2019) Transient central diabetes insipidus after discontinuation of vasopressin. Case Rep Endocrinol 2019:4189525
Gittoes NJL, Sheppard MC, Johnson AP, Stewart PM (1999) Outcome of surgery for acromegaly—the experience of a dedicated pituitary surgeon. QJM 92:741–745
Jenkins D, O’Brien I, Johnson A et al (1995) The Birmingham pituitary database: auditing the outcome of the treatment of acromegaly. Clin Endocrinol 43:517–522
Swearingen B (1998) Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J Clin Endocrinol Metab 83:3419–3426
Fusco A, Zatelli MC, Bianchi A et al (2008) Prognostic significance of the Ki-67 labeling index in growth hormone-secreting pituitary adenomas. J Clin Endocrinol Metab 93:2746–2750
Widhalm G, Wolfsberger S, Preusser M et al (2009) Residual nonfunctioning pituitary adenomas: prognostic value of MIB-1 labeling index for tumor progression. J Neurosurg 111:563–571
Shimura T, Kofunato Y, Okada R et al (2016) MIB-1 labeling index, Ki-67, is an indicator of invasive intraductal papillary mucinous neoplasm. Mol Clin Oncol 5:317–322
Byun S-S, Lee M, Hong SK, Lee H (2019) Elevated Ki-67 (MIB-1) expression as an independent predictor for unfavorable pathologic outcomes and biochemical recurrence after radical prostatectomy in patients with localized prostate cancer: A propensity score matched study. PLoS ONE 14:e0224671
Shahrestani S, Ballatori AM, Chen XT et al (2020) Analysis of modifiable and nonmodifiable risk factors in patients undergoing pituitary surgery. J Neurosurg. https://doi.org/10.3171/2020.4.JNS20417
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahrestani, S., Cardinal, T., Micko, A. et al. Neural network modeling for prediction of recurrence, progression, and hormonal non-remission in patients following resection of functional pituitary adenomas. Pituitary 24, 523–529 (2021). https://doi.org/10.1007/s11102-021-01128-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-021-01128-5