Abstract
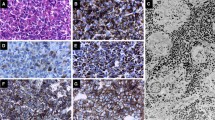
Ghrelin, an orexigenic hormone, is known to occur in the normal anterior pituitary where its physiologic role is uncertain but may include promotion of appetite. We sought to investigate anticipated differences in adenohypophysial and neurohypophysial ghrelin immunoexpression between normal subjects and patients with anorexia nervosa who had succumbed to complications of the disease. We hypothesized that the glands of anorexia nervosa patients would show relative diminished action in ghrelin content. The study included 12 autopsy-derived pituitaries of anorexia nervosa and 10 control glands. The streptavidin-biotin-peroxidase complex method and double immunohistochemical staining method were used to determine which cell types expressed both ghrelin and adenohypophysial hormones. Nontumorous control pituitaries were also obtained at autopsy. In anorexia nervosa and control adenohypophyses, ghrelin was mainly localized in somatotrophs and to a lesser extent in corticotrophs and gonadotrophs. Ghrelin accumulated within nerve fibers and Herring bodies in the neurohypophysis and pituitary stalk. In the controls, ghrelin expression was apparent in only a few cases. It was mild and only along few nerve fibers. In the adenohypophyses of anorexia nervosa patients, ghrelin was not depleted. It appears that in these patients, ghrelin is transported in excess from the hypothalamic neurohypophysial tract to the neurohypophysis.




Similar content being viewed by others
References
Walsh BT (2008) Eating disorders. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J (eds) Harrison’s principles of internal medicine, 17th edn. McGraw-Hill Medical, New York
Nogal P, Pniewska-Siark B, Lewiński A (2008) Evaluation of selected clinical and diagnostic parameters in girls with anorexia nervosa (I). Neuro Endocrinol Lett 29:421–427
Himmerich H, Schönknecht P, Heitmann S, Sheldrick AJ (2010) Laboratory parameters and appetite regulators in patients with anorexia nervosa. J Psychiatr Pract 16:82–92
Hoek HW, van Hoeken D (2003) Review of the prevalence and incidence of eating disorders. Int J Eat Disord 34:383–396
Keel PK, Dorer DJ, Eddy KT, Franko D, Charatan DL, Herzog DB (2003) Predictors of mortality in eating disorders. Arch Gen Psychiatry 60:179–183
Birmingham CL, Su J, Hlynsky JA, Goldner EM, Gao M (2005) The mortality rate from anorexia nervosa. Int J Eat Disord 38:143–146
Messini CI, Dafopoulos K, Chalvatzas N, Georgoulias P, Messinis IE (2009) Effect of ghrelin on gonadotrophin secretion in women during the menstral cycle. Hum Pathol 24:976–981
Lawson EA, Klibanski A (2008) Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab 4:407–414
Kluge M, Biedl S, Uhr M, Schmidt D, Zhang X, Yassouridis A, Steiger A (2010) Ghrelin affects the hypothalamus-pituitary-thyroid axis in humans by increasing free thyroxine and decreasing TSH in plasma. Eur J Endocrinol 162:1059–1065
Broglio F, Gottero C, Arvat E, Ghigo E (2003) Endocrine and non-endocrine actions of ghrelin. Horm Res 59:109–117
Tolle V, Kadem M, Bluet-Pajot MT, Frere D, Foulon C, Bossu C, Dardennes R, Mounier C, Zizzri P, Lang F, Epelbaum J, Estour B (2003) Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J Clin Endocrinol Metab 88:109–116
Støving RK, Chen JW, Glintborg D, Brixen K, Flyvbjerg A, Hørder K, Frystyk J (2007) Bioactive insulin-like growth factor (IGF) 1 and IGF-binding protein-1 in anorexia nervosa. J Clin Endocrinol Metab 92:2323–2329
Misra M, Klibanski A (2010) Neuroendocrine consequences of anorexia nervosa in adolescents. Endocr Dev 17:197–214
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660
Tomasetto C, Karam SM, Ribieras S, Masson R, Lefèbvre O, Staub A, Alexander G, Chenard MP, Rio MC (2000) Identification and characterization of a novel gastric peptide hormone: the motilin-related peptide. Gastroenterology 119:395–405
Gualillo O, Caminos J, Blanco M, Garcia-Caballero T, kojima M, Kangawa K, Dieguez C, Casanueva F (2001) Ghrelin, a novel placental-derived hormone. Endocrinology 142:788–794
Mori K, Yoshimoto A, Takaya K, Hosoda K, Ariyasi H, Yahata K, Mukoyama M, Sugawara A, Hosoda H, Kojima M, Kangawa K, Nakao K (2000) Kidney produces a novel acylated peptide, ghrelin. FEBS Lett 486:213–216
Tena-sempere M, Barreiro ML, González LC, Gaytán F, Zhang FP, Caminos JE, Pinilla L, Casanueva FF, Diéguez C, Aguilar E (2002) Novel expression and functional role of ghrelin in rat testis. Endocrinology 143:717–725
Lu S, Guan JL, Wang QP, Uehara K, Yamada S, Goto N, Date Y, Nakazato M, Kojima M, Kangawa K, Shioda S (2002) Immunocytochemical observation of ghrelin-containing neurons in the rat arcuate nucleus. Neurosci Lett 321:157–160
Rotondo F, Cusimano M, Scheithauer BW, Rotondo A, Syro LV, Kovacs K (2011) Ghrelin immunoexpression in pituitary adenomas. Pituitary 14:318–322
Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR (2001) Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86:5992
Cummings DE (2006) Ghrelin and the short-and long-term regulation of appetite and body weight. Physiol Behav 89:71–84
Korbonits M, Kojima M, Kangawa K, Grossman AB (2001) Presence of ghrelin in normal and adenomatous human pituitary. Endocrine 14:101–104
Janas-Kozik M, Krupa-Matuszczyk I, Tomasik-Krótki J (2006) Ghrelin- the guardian of energy balance. Psychiatr Pol 40:119–128
Soriano-Guillen L, Barrios V, Campos-Barros A, Argente J (2004) Ghrelin levels in obesity and anorexia nervosa: effect of weight reduction or recuperation. J Pediatr 144:36–42
Shimizu Y, Nagaya N, Isobe T, Imazu M, Okumura H, Hosoda H, Kojima M, Kangawa K, Kohno N (2003) Increased plasma ghrelin level in lung cancer cachexia. Clin Cancer Res 9:774–778
Garcia JM, Garcia-Touza M, Hijazi RA, Taffet G, Epner D, Mann D, Smith RG, Cunningham GR, Marcelli M (2005) Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab 90:2920–2926
Nakahara T, Harada T, Yasuhara D, Shimada N, Amitani H, Sakoguchi T, Kamiji MM, Asakawa A, Inui A (2008) Plasma obestatin concentrations are negatively correlated with body mass index, insulin resistance index, and plasma leptin concentrations in obesity and anorexia nervosa. Biol Psychiatry 64:252–255
Yakabi K, Sadakane C, Noguchi M, Ohno S, Ro S, Chinen K, Aoyama T, Sakurada T, Takabayashi H, Hattori T (2010) Reduced ghrelin secretion in the hypothalamus of rats due to cisplatin-induced anorexia. Endocrinology 151:3773–3782
Scheithauer BW, Kovacs K, Jariwala LK, Randall RV, Ryan N (1988) Anorexia nervosa: an immunohistochemical study of the pituitary gland. Mayo Clin Proc 63:23–28
Rotondo F, Scheithauer BW, Kovacs K, Bell DC (2009) Rab 3B immunoexpression in human pituitary adenomas. Appl Immunohistochem Mol Morphol 17:185–188
Rotondo F, Kovacs K, Horvath E, Bell CD, Lloyd RV, Scheithauer BW (2006) Immunohistochemical expression of nestin in the non-tumorous hypophysis and in pituitary neoplasms. Acta Neuropathol 111:272–277
Nakagawa SA, Lopes A, Lopes de Carvalho A, Rossi BM, Werneck da Cunha I, Soares FA, Chung WT, Alves LA (2010) Nitric oxide synthases, cyclooxygenase-2, nitrotyrosine and angiogenesis in chondrosarcoma and their relation to prognosis. J Bone Joint Surg Am 92:1738–1746
Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M (2000) Ghrelin, a novel growth-hormone releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141:4255–4261
Rindi G, Necchi V, Savio A, Torsello A, Zoli M, Locatelli V, Raimondo F, Cocchi D, Solcia E (2002) Characterization of gastric ghrelin cells in man and other mammals: studies in adults and fetal tissues. Histochem Cell Biol 117:511–519
Rindi G, Torsello A, Locatelli V, Solcia E (2004) Ghrelin expression and actions: a novel peptide for an old cell type of the diffuse endocrine system. Exp Biol Med 229:1007–1016
Nagaya N, Kojima M, Uematsu M, Yamagishi M, Hosoda H, Oya H, Hayashi Y, Kangawa K (2001) Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am J Physiol Regul Integr Comp Physiol 280:R1483–R1487
Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S (2001) Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun 280:904–907
Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E (2001) Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab 86:5083–5086
Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K (2000) Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab 85:4908–4911
Shiiya T, Nakazato M, Mizuta M, Mondal SM, Nakazato M (2002) Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab 87:240–244
Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, Hayashi T, Inoue G, Hosoda K, Kojima M, Kangawa K, Nakao K (2001) Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes 50:227–232
Kojima M, Hosoda H, Kangawa K (2004) Clinical endocrinology and metabolism. Ghrelin, a novel growth hormone-releasing and appetite-stimulating peptide from stomach. Best Pract Res Clin Endocrinol Metab 18:517–530
Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T, Guan JL, Wang QP, Funahashi H, Sakurai T, Shioda S, Matsukura S, Kangawa K, Nakazato M (2003) Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 144:1506–1512
Funahashi H, Takenoya F, Guan JL, Kageyama H, Yada T, Shioda S (2003) Hypothalamic neuronal networks and feeding-related peptides involved in the regulation of feeding. Anat Sci Int 78:123–138
Amar AP, Weiss MH (2003) Pituitary anatomy and physiology. Neurosurg Clin North Am 14:11–23
Takahashi K, Murakami O, Satoh F, Mouri T (2002) The hypothalamus and neurohypophysis. In: Stefaneanu L, Sasano H, Kovacs K (eds) Molecular and cellular endocrine pathology. Arnold, London, pp 45–74
Mouri T, Itoi K, Takahashi K, Suda T, Murakami O, Yoshinaga K, Andoh N, Ohtani H, Masuda T, Sasano N (1993) Colocalization of corticotropin-releasing factor and vasopressin in the paraventricular nucleus of the human hypothalamus. Neuroendocrinology 57:34–39
Angelidis G, Valotassiou V, Georgoulias P (2010) Current and potential roles of ghrelin in clinical practice. J Endocrinol Invest 33:823–838
Acknowledgments
The Authors wish to express their sincere gratitude to the Jarislowsky and Lloyd Carr-Harris Foundations for their generous support and to Mrs. Denise Chase for her expert secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rotondo, F., Scheithauer, B.W., Syro, L.V. et al. Pituitary immunoexpression of ghrelin in anorexia nervosa. Pituitary 15, 533–538 (2012). https://doi.org/10.1007/s11102-011-0364-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-011-0364-6