Abstract
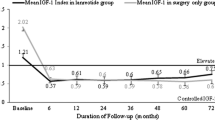
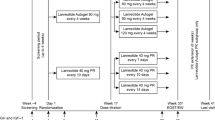
We investigated the effectiveness of lanreotide for the treatment of active acromegaly in a retrospectively multicenter case series including 53 patients (24 male, 29 female; mean age at diagnosis, 49.5 ± 13.9 years) with acromegaly treated with lanreotide in nine different centers. Mean tumor diameter was 20 ± 13 mm; mean basal levels of growth hormone (GH) and insulin-like growth factor I (IGF-I) were 21.3 ± 26.3 and 579 ± 177 μg/l, respectively. The primary mode of treatment was surgery in 70% of patients. Twenty-nine patients received only lanreotide (Prolonged Release, Autogel), whereas 24 subjects were also treated with octreotide at another treatment stage. Primary therapy with lanreotide was administered in five patients. Maximal monthly dose of lanreotide Autogel (n = 44) was 60 mg in 45%, 90 mg in 26%, 120 mg in 21% and 180 mg in 8%. During 36 months of lanreotide treatment, mean IGF-I levels decreased from 443 ± 238 to 276 ± 147 μg/l (P < 0.001), and mean GH levels, from 5.2 ± 6.4 to 3.2 ± 3.0 μg/l (P < 0.001). IGF-I levels normalized in 51% of patients and decreased by >50% towards normal in 32%; the normalization rate was higher in women (65%) than men (33%, P = 0.04). Safe random GH levels (≤2 μg/l) were achieved in 49% of patients. Both IGF-I normalization and safe GH levels were reached in 32% of the cohort. Lanreotide is an effective treatment for active acromegaly. Female sex was associated with higher rates of IGF-I normalization.


Similar content being viewed by others
References
Holdaway IM, Rajasoorya RC, Gamble GD (2004) Factors influencing mortality in acromegaly. J Clin Endocrinol Metab 89:667–674. doi:10.1210/jc.2003-031199
Kauppinen-Makelin R, Sane T, Reunanen A, Valimaki MJ, Niskanen L, Markkanen H, Loyttyniemi E, Ebeling T, Jaatinen P, Laine H, Nuutila P, Salmela P, Salmi J, Stenman UH, Viikari J, Voutilainen E (2005) A nationwide survey of mortality in acromegaly. J Clin Endocrinol Metab 90:4081–4086. doi:10.1210/jc.2004-1381
Rajasoorya C, Holdaway IM, Wrightson P, Scott DJ, Ibbertson HK (1994) Determinants of clinical outcome and survival in acromegaly. Clin Endocrinol (Oxf) 41:95–102. doi:10.1111/j.1365-2265.1994.tb03789.x
Biermasz NR, Dekker FW, Pereira AM, van Thiel SW, Schutte PJ, van Dulken H, Romijn JA, Roelfsema F (2004) Determinants of survival in treated acromegaly in a single center: predictive value of serial insulin-like growth factor I measurements. J Clin Endocrinol Metab 89:2789–2796. doi:10.1210/jc.2003-032041
Swearingen B, Barker FGII, Katznelson L, Biller BM, Grinspoon S, Klibanski A, Moayeri N, Black PM, Zervas NT (1998) Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J Clin Endocrinol Metab 83:3419–3426. doi:10.1210/jc.83.10.3419
Melmed S (2006) Acromegaly. N Engl J Med 355:2558–2573. doi:10.1056/NEJMra062453
Heron I, Thomas F, Dero M, Gancel A, Ruiz JM, Schatz B, Kuhn JM (1993) Pharmacokinetics and efficacy of a long-acting formulation of the new somatostatin analog BIM 23014 in patients with acromegaly. J Clin Endocrinol Metab 76:721–727. doi:10.1210/jc.76.3.721
Caron P, Beckers A, Cullen DR, Goth MI, Gutt B, Lauberg P, Pico AH, Valimaki M, Zgliczynsk W (2002) Efficacy of the new long-acting formulation of lanreotide (lanreotide Autogel) in the management of acromegaly. J Clin Endocrinol Metab 87:99–104. doi:10.1210/jc.87.1.99
Lancranjan I, Bruns C, Grass P, Jaquet P, Jervell J, Kendall-Taylor P, Lamberts SW, Marbach P, Orskov H, Pagani G, Sheppard M, Simionescu L (1996) Sandostatin LAR: a promising therapeutic tool in the management of acromegalic patients. Metabolism 45:67–71. doi:10.1016/S0026-0495(96)90087-6
Freda PU (2002) Somatostatin analogs in acromegaly. J Clin Endocrinol Metab 87:3013–3018. doi:10.1210/jc.87.7.3013
Caron P, Cogne M, Raingeard I, Bex-Bachellerie V, Kuhn JM (2006) Effectiveness and tolerability of 3-year lanreotide Autogel treatment in patients with acromegaly. Clin Endocrinol (Oxf) 64:209–214. doi:10.1111/j.1365-2265.2006.02450.x
Melmed S, Casanueva FF, Cavagnini F, Chanson P, Frohman L, Grossman A, Ho K, Kleinberg D, Lamberts S, Laws E, Lombardi G, Vance ML, Werder KV, Wass J, Giustina A (2002) Acromegaly treatment consensus workshop participants. Guidelines for acromegaly management. J Clin Endocrinol Metab 87:4054–4058. doi:10.1210/jc.2002-011841
Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, Veldhuis J, Wass J, Von Werder K, Melmed S (2000) Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab 85:526–529. doi:10.1210/jc.85.2.526
Ronchi CL, Varca V, Giavoli C, Epaminonda P, Beck-Peccoz P, Spada A, Arosio M (2005) Long-term evaluation of postoperative acromegalic patients in remission with previous and newly proposed criteria. J Clin Endocrinol Metab 90:1377–1382. doi:10.1210/jc.2004-1974
Schonbrunn A (1999) Somatostatin receptors present knowledge and future directions. Ann Oncol 10(Suppl 2):S17–S21. doi:10.1023/A:1027392228418
Shimon I, Melmed S (1997) Structure and function of somatostatin receptors in growth hormone control. J Endocrinol 155(Suppl 1):S3–S6 (discussion S7–S8)
Panetta R, Patel YC (1995) Expression of mRNA for all five human somatostatin receptors (hSSTR1–5) in pituitary tumors. Life Sci 56:333–342. doi:10.1016/0024-3205(94)00956-2
Miller GM, Alexander JM, Bikkal HA, Katznelson L, Zervas NT, Klibanski A (1995) Somatostatin receptor subtype gene expression in pituitary adenomas. J Clin Endocrinol Metab 80:1386–1392. doi:10.1210/jc.80.4.1386
Greenman Y, Melmed S (1994) Expression of three somatostatin receptor subtypes in pituitary adenomas: evidence for preferential SSTR5 expression in the mammosomatotroph lineage. J Clin Endocrinol Metab 79:724–729. doi:10.1210/jc.79.3.724
Greenman Y, Melmed S (1994) Heterogeneous expression of two somatostatin receptor subtypes in pituitary tumors. J Clin Endocrinol Metab 78:398–403. doi:10.1210/jc.78.2.398
Heron I, Thomas F, Dero M, Poutrain JR, Henane S, Catus F, Kuhn JM (1993) Treatment of acromegaly with sustained-release lanreotide, a new somatostatin analog. Presse Med 22:526–531
Stewart PM, Kane KF, Stewart SE, Lancranjan I, Sheppard MC (1995) Depot long-acting somatostatin analog (Sandostatin LAR) is an effective treatment for acromegaly. J Clin Endocrinol Metab 80:3267–3272. doi:10.1210/jc.80.11.3267
Attanasio R, Baldelli R, Pivonello R, Grottoli S, Bocca L, Gasco V, Giusti M, Tamburrano G, Colao A, Cozzi R (2003) Lanreotide 60 mg, a new long-acting formulation: effectiveness in the chronic treatment of acromegaly. J Clin Endocrinol Metab 88:5258–5265. doi:10.1210/jc.2003-030266
Ronchi CL, Boschetti M, Degli Uberti EC, Mariotti S, Grottoli S, Loli P, Lombardi G, Tamburrano G, Arvigo M, Angeletti G, Boscani PF, Beck-Peccoz P, Arosio M (2007) Efficacy of a slow-release formulation of lanreotide (Autogel® 120 mg) in patients with acromegaly previously treated with octreotide long acting release (LAR): an open, multicenter longitudinal study. Clin Endocrinol (Oxf) 67:512–519
Baldelli R, Colao A, Razzore P, Jaffrain-Rea ML, Marzullo P, Ciccarelli E, Ferretti E, Ferone D, Gaia D, Camanni F, Lombardi G, Tamburrano G (2000) Two-year follow-up of acromegalic patients treated with slow release lanreotide (30 mg). J Clin Endocrinol Metab 85:4099–4103. doi:10.1210/jc.85.11.4099
Freda PU, Katznelson L, van der Lely AJ, Reyes CM, Zhao S, Rabinowitz D (2005) Long-acting somatostatin analog therapy of acromegaly: a meta-analysis. J Clin Endocrinol Metab 90:4465–4473. doi:10.1210/jc.2005-0260
Murray RD, Melmed S (2008) A critical analysis of clinically available somatostatin analog formulations for therapy of acromegaly. J Clin Endocrinol Metab 8:2957–2968. doi:10.1210/jc.2008-0027
Chanson P, Borson-Chazot F, Kuhn J-M, Blumberg J, Maisonobe P, Delemer B (2008) Control of IGF-I levels with titrated dosing of lanreotide Autogel over 48 weeks in patients with acromegaly. Clin Endocrinol (Oxf) 69:299–305. doi:10.1111/j.1365-2265.2008.03208.x
Bates AS, Evans AJ, Jones P, Clayton RN (1995) Assessment of GH status in acromegaly using serum growth hormone, serum insulin-like growth factor-1 and urinary growth hormone excretion. Clin Endocrinol (Oxf) 42:417–423. doi:10.1111/j.1365-2265.1995.tb02651.x
Wass JAH (1997) Growth hormone, insulin-like growth factor-I and its binding proteins in the follow-up of acromegaly. J Endocrinol 155(Suppl 1):S17–S19
Freda PU, Post KD, Powell JS, Wardlaw SL (1998) Evaluation of disease status with sensitive measures of growth hormone secretion in 60 postoperative patients with acromegaly. J Clin Endocrinol Metab 83:3808–3816. doi:10.1210/jc.83.11.3808
Growth Hormone Research Society, Pituitary Society (2004) Biochemical assessment and long-term monitoring in patients with acromegaly: statement from a joint consensus conference of the growth hormone research society and the pituitary society. J Clin Endocrinol Metab 89:3099–3102. doi:10.1210/jc.2003-031138
Alexopoulou O, Bex M, Abs R, T’Sjoen G, Velkeniers B, Maiter D (2008) Divergence between growth hormone and insulin-like growth factor-I concentrations in the follow-up of acromegaly. J Clin Endocrinol Metab 93:1324–1330. doi:10.1210/jc.2007-2104
Freda PU (2000) Advances in the diagnosis of acromegaly. Endocrinologist 10:237–244. doi:10.1097/00019616-200010040-00005
Holdaway IM, Bolland MJ, Gamble GD (2008) A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol 159:89–95. doi:10.1530/EJE-08-0267
Parkinson C, Ryder WDJ, Trainer PJ (2001) The relationship between serum GH and serum IGF-I in acromegaly is gender-specific. J Clin Endocrinol Metab 86:5240–5244. doi:10.1210/jc.86.11.5240
Weissberger AJ, Ho KK, Lazarus L (1991) Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women. J Clin Endocrinol Metab 72:374–381
Engstrom BE, Burman P, Karlsson FA (2002) Men with acromegaly need higher doses of octreotide than women. Clin Endocrinol (Oxf) 56:73–77. doi:10.1046/j.0300-0664.2001.01440.x
Colao A, Pivonello R, Cappabianca P, Briganti F, Tortora F, Auriemma RS, De Martino MC, Marzullo P, Lombardi G (2005) Effect of gender and gonadal status on the long-term response to somatostatin analogue treatment in acromegaly. Clin Endocrinol (Oxf) 63:342–349. doi:10.1111/j.1365-2265.2005.02351.x
Dobrashian RD, O’Halloran DJ, Hunt A, Beardwell CG, Shalet SM (1993) Relationships between insulin-like growth factor-1 levels and growth hormone concentration during diurnal profiles and following oral glucose in acromegaly. Clin Endocrinol (Oxf) 38:589–593. doi:10.1111/j.1365-2265.1993.tb02139.x
Colao A, Pivonello R, Auriemma RS, Briganti F, Galdiero M, Tortora F, Caranci F, Cirillo S, Lombardi G (2006) Predictors of tumor shrinkage after primary therapy with somatostatin analogs in acromegaly: a prospective study in 99 patients. J Clin Endocrinol Metab 91:2112–2118. doi:10.1210/jc.2005-2110
Chanson P, Boerlin V, Ajzenberg C, Bachelot Y, Benito P, Bringer J, Caron P, Charbonnel B, Cortet C, Delemer B, Escobar-Jimenez F, Foubert L, Gaztambide S, Jockenhoevel F, Kuhn JM, Leclere J, Lorcy Y, Perlemuter L, Prestele H, Roger P, Rohmer V, Santen R, Sassolas G, Scherbaum WA, Schopohl J, Torres E, Varela C, Villamil F, Webb SM (2000) Comparison of octreotide acetate LAR and lanreotide SR in patients with acromegaly. Clin Endocrinol (Oxf) 53:577–586. doi:10.1046/j.1365-2265.2000.01134.x
Turner HE, Vadivale A, Keenan J, Wass JA (1999) A comparison of lanreotide and octreotide LAR for treatment of acromegaly. Clin Endocrinol (Oxf) 51:275–280. doi:10.1046/j.1365-2265.1999.00853.x
Cozzi R, Dallabonzana D, Attanasio R, Barausse M, Oppizzi G (1999) A comparison between octreotide-LAR and lanreotide-SR in the chronic treatment of acromegaly. Eur J Endocrinol 141:267–271. doi:10.1530/eje.0.1410267
van Thiel SW, Romijn JA, Biermasz NR, Ballieux BE, Frolich M, Smit JW, Corssmit EP, Roelfsema F, Pereira AM (2004) Octreotide long-acting repeatable and lanreotide Autogel are equally effective in controlling growth hormone secretion in acromegalic patients. Eur J Endocrinol 150:489–495. doi:10.1530/eje.0.1500489
Ashwell SG, Bevan JS, Edwards OM, Harris MM, Holmes C, Middleton MA, James RA (2004) The efficacy and safety of lanreotide Autogel in patients with acromegaly previously treated with octreotide LAR. Eur J Endocrinol 150:473–480. doi:10.1530/eje.0.1500473
Alexopoulou O, Abrams P, Verhelst J, Poppe K, Velkeniers B, Abstract R, Maiter D (2004) Efficacy and tolerability of lanreotide Autogel therapy in acromegalic patients previously treated with octreotide LAR. Eur J Endocrinol 151:317–324. doi:10.1530/eje.0.1510317
Amato G, Mazziotti G, Rotondi M, Iorio S, Doga M, Sorvillo F, Manganella G, Di Salle F, Giustina A, Carella C (2002) Long-term effects of lanreotide SR and octreotide LAR® on tumour shrinkage and GH hypersecretion in patients with previously untreated acromegaly. Clin Endocrinol (Oxf) 56:65–71. doi:10.1046/j.0300-0664.2001.01438.x
Gutt B, Bidlingmaier M, Kretschmar K, Dieterle C, Steffin B, Schopohl J (2005) Four-year follow-up of acromegalic patients treated with the new long-acting formulation of lanreotide (Lanreotide Autogel). Exp Clin Endocrinol Diabetes 113:139–144. doi:10.1055/s-2005-837520
Acknowledgments
This study was partially supported by a grant from Medison Pharma, Israel. We thank Ms. Gloria Ginzach for her assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toledano, Y., Rot, L., Greenman, Y. et al. Efficacy of long-term lanreotide treatment in patients with acromegaly. Pituitary 12, 285–293 (2009). https://doi.org/10.1007/s11102-009-0172-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-009-0172-4