Abstract
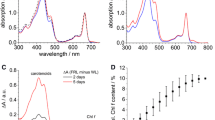
Halomicronema hongdechloris is a chlorophyll (Chl) f-producing cyanobacterium. Chl f biosynthesis is induced under far-red light, extending its photosynthetically active radiation range to 760 nm. In this study, PSI complexes were isolated and purified from H. hongdechloris, grown under white light (WL) and far-red light (FR), by a combination of density gradient ultracentrifugation and chromatographic separation. WL-PSI showed similar pigment composition as that of Synechocystis 6803, using Chl a in the reaction center. Both Chl a and f were detected in the FR-PSI, although Chl f was a minor component (~8% of total Chl). The FR-PSI showed a maximal fluorescence emission peak of 750 nm at 77 K, which is red-shifted ~20 nm compared to the 730 nm recorded from the WL-PSI. The absorption peaks of P700 for WLPSI and FR-PSI were 699 nm and 702 nm, respectively. The function of Chl f in FR-PSI is discussed.
Similar content being viewed by others
Abbreviations
- APC:
-
allophycocyanin
- CCA:
-
complementary chromatic adaptation
- Chl:
-
chlorophyll
- FR:
-
730 nm light-emitting diodes
- DoDM:
-
β-dodecyl maltoside
- FaRLiP:
-
far-red light photoacclimation
- FR:
-
far-red light
- FR-PSI:
-
isolated PSI from 730 nm LED-illuminated culture
- OG:
-
n-octyl-β-D-glucopyranoside
- PC:
-
phycocyanin
- TMPD:
-
N,N,N',N'-tetramethyl-p-phenylenediamine
- WL:
-
white fluorescent light
- WL-PSI:
-
isolated PSI from white light culture
- 6803-PSI:
-
purified PSI complexes from Synechocystis 6803
References
Airs R.L., Temperton B., Sambles C. et al.: Chlorophyll f and chlorophyll d are produced in the cyanobacterium Chlorogloeopsis fritschii when cultured under natural light and nearinfrared radiation.–FEBS Lett. 588: 3770–3777, 2014.
Akutsu S., Fujinuma D., Furukawa H. et al.: Pigment analysis of a chlorophyll f-containing cyanobacterium strain KC1isolated from Lake Biwa.–Photochem. Photobiol. 33: 35–40, 2011.
Amunts A., Toporik H., Borovikova A. et al.: Structure determination and improved model of plant photosystem I.–J. Biol. Chem. 285: 3478–3486, 2010.
Barber J.: Photosynthetic generation of oxygen.–Philos. T. R. Soc. B 363: 2665–2674, 2008.
Barth P., Lagoutte B., Sétif P.: Ferredoxin reduction by photosystem I from Synechocystis sp. PCC 6803: toward an understanding of the respective roles of subunits PsaD and PsaE in ferredoxin binding.–Biochemistry 37: 16233–16241, 1998.
Ben-Shem A., Frolow F., Nelson N.: Crystal structure of plant photosystem I.–Nature 426: 630–635, 2003.
Chen M., Blankenship R.: Expanding the solar spectrum used by photosynthesis.–Trends Plant Sci. 16: 427–431, 2011.
Chen M., Li Y., Birch D. et al: A cyanobacterium that contains chlorophyll f–a red-absorbing photopigment.–FEBS Lett. 586: 3249–3254, 2012.
Chen M., Schliep M., Willows R. et al: A red-shifted chlorophyll.–Science 329: 1318–1319, 2010.
Croce R., van Amerongen H.: Light-harvesting in photosystem I.–Photosynth. Res. 116: 153–166, 2013.
El-Khouly M.E., El-Mohsnawy E., Fukuzumi S.: Solar energy conversion: From natural to artificial photosynthesis.–J. Photoch. Photobio. C 31: 36–83, 2017.
El-Mohsnawy E., Kopczak M.J., Schlodder E. et al.: Structure and function of intact photosystem I monomers from the cyanobacterium Thermosynechococcus elongatus.–Biochemistry49: 4740–4751, 20
Fromme P., Jordan P., Krauß N.: Structure of photosystem I.–BBA-Bioenergetics 1507: 5–31, 2001.
Gan F., Bryant D.A.: Adaptive and acclimative responses of cyanobacteria to far-red light.–Environ. Microbiol. 17: 3450–3465, 2015.
Gan F., Zhang S., Rockwell N.C. et al.: Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light.–Science 345: 1312–1317, 2014.
Golbeck, J.H.: Photosystem I in cyanobacteria.–In: Bryant D.A. (ed.): The Molecular Biology of Cyanobacteria. Pp. 319–360. Springer, Dordrecht 1994.
Golub M., Hejazi M., Kölsch A. et al.: Solution structure of monomeric and trimeric photosystem I of Thermosynechococcus elongatus investigated by small-angle X-ray scattering.–Photosynth. Res. 133: 163–173, 2017.
Goodwint W.: Biochemistry of pigments.–In Waterman T.H. (ed.): The Physiology of Crustacea. Pp. 101–140. Academic Press, New York and London 1960.
Grotjohann I., Fromme P.: Structure of cyanobacterial photosystem I.–Photosynth. Res. 85: 51–72, 2005.
Hiyama T., Ke B.: Difference spectra and extinction coefficients of P 700.–BBA-Bioenergetics. 267: 160–171, 1972.
Hou H.J., Allakhverdiev S.I., Najafpour M.M. et al.: Current challenges in photosynthesis: from natural to artificial.–Front Plant Sci. 5: 232, 2014.
Hu Q., Miyashita H., Iwasaki I. et al: A photosystem I reaction center driven by chlorophyll d in oxygenic photosynthesis.–P. Natl. Acad. Sci. USA 95: 13319–13323, 1998.
Jordan P., Fromme P., Witt H.T. et al.: Three-dimensional structure of cyanobacterial photosystem I at 2.5 angstrom resolution.–Nature 411: 909–917, 2001.
Kruip J., Boekema E.J., Bald D. et al.: Isolation and structural characterization of monomeric and trimeric photosystem I complexes (P700. FA/FB and P700. FX) from the cyanobacterium Synechocystis PCC 6803.–J. Biol. Chem. 268: 23353–23360, 1993.
Li M, Semchonok D.A., Boekema E.J., Bruce B.D.: Characterization and evolution of tetrameric photosystem I from the thermophilic cyanobacterium Chroococcidiopsis sp TS-821.–Plant Cell 26: 1230–1245, 2014.
Li Y., Cai Z.-L. Chen M.: Spectroscopic properties of chlorophyll f.–J. Phys. Chem. B 117: 11309–11317, 2013.
Li Y., Chen M.: Novel chlorophylls and new directions in photosynthesis research.–Funct. Plant Biol. 42: 493–501, 2015.
Li Y., Lin Y., Garvey C.J. et al.: Characterization of red-shifted phycobilisomes isolated from the chlorophyll f-containing cyanobacterium Halomicronema hongdechloris.–BBABioenergetics 1857: 107–114, 20
Li Y., Lin Y., Loughlin P. et al.: Optimization and effects of different culture conditions on growth of Halomicronema hongdechloris–a filamentous cyanobacterium containing chlorophyll f.–Front. Plant Sci. 5: 67, 2014.
Li Y., Scales N., Blankenship R. E. et al.: Extinction coefficient for red-shifted chlorophylls: chlorophyll d and chlorophyll f.–BBA-Bioenergetics 1817: 1292–1298, 2012.
Miyashita H., Ikemoto H., Kurano N. et al.: Chlorophyll d as a major pigment.–Nature 383: 402, 1996.
Mühlenhoff U., Zhao J., Bryant D.A.: Interaction between photosystem I and flavodoxin from the cyanobacterium Synechococcus sp. PCC 7002 as revealed by chemical crosslinking.–Eur. J. Biochem. 235: 324–331, 1996.
Nelson N., Junge W.: Structure and energy transfer in photosystems of oxygenic photosynthesis.–Annu. Rev. Biochem. 84: 659–683, 2015.
Nyhus K.J., Ikeuchi M., Inoue Y. et al.: Purification and characterization of the photosystem I complex from the filamentous cyanobacterium Anabaena variabilis ATCC 29413.–J. Biol. Chem. 267: 12489–12495, 1992.
Ohkubo S., Miyashita H.: A niche for cyanobacteria producing chlorophyll f within a microbial mat.–ISME J. 11: 2368–2378, 2017.
Rögner M., Nixon P.J., Diner B.A.: Purification and characterization of photosystem I and photosystem II core complexes from wild-type and phycocyanin-deficient strains of the cyanobacterium Synechocystis PCC 6803.–J. Biol. Chem. 265: 6189–6196, 1990.
Schluchter W.M., Shen G., Zhao J., Bryant D.A.: Characterization of psal and psaL mutants of Synechococcus sp. strain PCC 7002: a new model for state transitions in cyanobacteria.–Photochem. Photobiol. 64: 53–66, 1996.
Sivakumar V., Wang R., Hastings G.: Photo-oxidation of P740, the primary electron donor in photosystem I from Acaryochloris marina.–Biophys. J. 85: 3162–3172, 20
Tomo T., Kato Y., Suzuki T. et al.: Characterization of highly purified photosystem I complexes from the chlorophyll ddominated cyanobacterium Acaryochloris marina MBIC 11017.–J. Biol. Chem. 283: 18198–18209, 2008.
Xu Q., Hoppe D., Chitnis V.P. et al.: Mutational analysis of photosystem I polypeptides in the cyanobacterium Synechocystis sp. PCC 6803. Targeted inactivation of psaI reveals the function of psaI in the structural organization of psaL.–J. Biol. Chem. 270: 16243–16250, 1995.
Xu W., Chitnis P., Valieva A. et al.: Electron transfer in cyanobacterial photosystem I: I. Physiological and spectroscopic characterization of site-directed mutants in a putative electron transfer pathway from A0 through A1 to FX.–J. Biol. Chem. 278: 27864–27875, 2003.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: The research was supported by the Australian Research Council Centre of Excellence for translational photosynthesis (CE140100015). The authors thank Dr. Ben Crossette (mass-spectral facility, University of Sydney) and Dr. Miguel A. Hernández-Prieto for assisting with LC-MS/MS and spectral analysis.
Electronic supplementary material
11099_2018_776_MOESM1_ESM.pdf
Fig. 1S. The HPLC chromatographs for the sucrose density band isolated from white light (WL) and far red light (FR) grown Halomicronema hongdechloris. The solid black (FR-2) and grey line (FR-1) represent the chromatographs for the FR band 2 and 1 (Fig.1), respectively. The dash black (WL-2) and grey line (WL-1) represent the chromatographs for the WL band 2 and 1 (Fig.1), respectively. Carotenoids, Chl f, Chl a, Chl a’, β-carotene and pheophytin a are assigned based on their retention time and online spectra.
11099_2018_776_MOESM2_ESM.pdf
Fig. 2S. Sequence alignment for PsaL’s detected from isolated PSI complexes of H. hongdechloris. PsaL (1JB0_L) from PSI crystal structure of Synechococcus elongates is used as a reference. The residues ligating chlorophyll a’s are highlighted by asterisk “*”.
Rights and permissions
About this article
Cite this article
Li, Y., Vella, N. & Chen, M. Characterization of isolated photosystem I from Halomicronema hongdechloris, a chlorophyll f-producing cyanobacterium. Photosynthetica 56, 306–315 (2018). https://doi.org/10.1007/s11099-018-0776-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-018-0776-x