Abstract
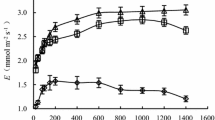
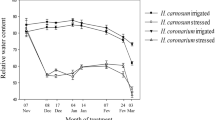
Caragana korshinskii Kom. is a perennial xerophytic shrub, well known for its ability to resist drought. In order to study ecophysiological responses of C. korshinskii under extreme drought stress and subsequent rehydration, diurnal patterns of gas exchange and chlorophyll (Chl) fluorescence parameters of photosystem II as well as Chl content were analyzed. Plant responses to extreme drought included (1) leaf abscission and using stem for photosynthesis, (2) improved instantaneous water-use efficiency, (3) decreased photosynthetic rate and partly closed stomata owing to leaf abscission and low water status, (4) decreased maximum photochemical efficiency of photosystem II (PSII) (variable to maximum fluorescence ratio, Fv/Fm), quantum efficiency of noncyclic electron transport of PSII, and Chl a and Chl b. Four days after rehydration, new leaves budded from stems. In the rewatered plants, the chloroplast function was restored, the gas exchange and Chl fluorescence returned to a similar level as control plant. The above result indicated that maintaining an active stem system after leaf abscission during extreme drought stress may be the foundation which engenders these mechanisms rapid regrowth for C. korshinskii in arid environment.
Similar content being viewed by others
Abbreviations
- C i :
-
intercellular CO2 concentration
- Chl:
-
chlorophyll
- DAW:
-
day after water was withheld
- DAR:
-
day after rehydration
- DS:
-
drought stress
- E :
-
transpiration rate
- F0 :
-
minimum fluorescence of the dark-adapted state
- Fm :
-
maximum fluorescence of the light-adapted state
- Fm′:
-
maximum fluoresence yield of the dark-adapted state
- Fs :
-
steady-state fluoresence
- Fv/Fm :
-
maximum photochemical efficiency of photosystem II
- FWC:
-
field water capacity
- g s :
-
stomatal conductance
- NPQ:
-
nonphotochemical quenching of fluorescence
- PPFD:
-
photosynthetic photon flux density
- P N :
-
net photosynthetic rate
- PSII:
-
photosystem II
- RWC:
-
relative water content
- SWC:
-
soil water content
- Tleaf :
-
leaf temperature
- WW:
-
well watered
- WUE:
-
instantaneous water-use efficiency
- ϕPSII :
-
quantum efficiency of noncyclic electric transport of PSII
References
Aganchich, B., Wahbi, S., Loreto, F., Centritto, M.: Partial root zone drying: regulation of photosynthetic limitations and antioxidant enzymatic act ivities in young olive (Olea europaea) saplings. — Tree Physiol. 29: 685–696, 2009.
Bai, J., Xu, D.H., Kang, H.M., Chen, K., Wang, G.: Photoprotective function of photorespiration in Reaumuria soongorica during different levels of drought stress in natural high irradiance. — Photosynthetica 46: 232–237, 2008.
Balaguer, L., Pugnaire, F.I., Martínez-Ferri, E.: Ecophysiological significance of chlorophyll loss and reduced photochemical efficiency under extreme aridity in Stipa tenacissima L. — Plant Soil 240: 343–352, 2002.
Boyer, T.S., Wong, S.C., Farquhar, D.: CO2 and water vapour exchange across the leaf cuticle (epidermis) at various water potentials. — Plant Physiol. 114: 185–191, 1997.
Chaitanya, K.V., Jutur, P.P., Sundar, D.: Water stress effects on photosynthesis in different mulberry cultivars. — Plant Growth Regul. 40: 75–80, 2003.
Cheng, X.R., Huang, M.B., Shao, M.A., Warrington, D.N.: A comparison of fine root distribution and water consumption of mature Caragana korshinkii Kom grown in two soils in a semiarid region, China. — Plant Soil 315: 149–161, 2009.
Comstock, J.P.: Hydraulic and chemical signaling in the control of stomatal conductance and transpiration. — J. Exp. Bot. 53: 195–200, 2002.
Cooper, K., Farrant, J.M.: Recovery of the resurrection plant Craterostigma wilmsii from desiccation: Protection versus repair. — J. Exp. Bot. 53: 1805–1813, 2002.
Croker, J.L., Witte, W.T., Auge, R.M.: Stomatal sensitivity of six temperate, deciduous tree species to non-hydraulic root-to shoot signalling of partial soil drying. — J. Exp. Bot. 49: 761–774, 1998.
Donald, R.: When there is too much light. — Plant Physiol. 125: 29–32, 2001.
Fang, X.W., Li, Y.B., Xu, D.H., Yang, X.M., Wang, G.: Activities of starch hydrolytic enzymes and starch mobilization in roots of Caragana korshinskii following aboveground partial shoot removal. — Trees 21: 93–100, 2007.
Farquhar, G.D., Sharkey, T.D.: Stomatal conductance and photosynthesis. — Ann. Rev. Plant Physiol. 33: 317–345, 1982.
Fleck, I., Hogan, K.P., Llorens, L., Abadía, A., Aranda, X.: Photosynthesis and photoprotection in Quercus ilex resprouts after fire. — Tree Physiol. 18: 607–614, 1998.
Gindaba, J., Rozanov, A., Negash, L.: Response of seedlings of two Eucalyptus and three deciduous tree species from Ethiopia to severe water stress. — Forest Ecol. Manag. 201: 119–129, 2004.
Genty, B., Briantais, J.M., Baker, N.R.: The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. — Biochim. Biophys. Acta 990: 87–92, 1989.
Guo, W.H., Li, B., Huang, Y.M.: Effects of different water stresses on eco-physiological characteristics of Hippophae rhamnoides seedlings. — Acta. Bot. Sin. 45: 1238–1244, 2003.
Kitao, M., Lei, T.T., Koike, T., Tobita, H., Maruyama, Y.: Higher electron transport rate observed at low intercellular CO2 concentration in long-term drought-acclimated leaves of Japanese mountain birch (Betula ermanii). — Physiol. Plant. 118: 406–413, 2003.
Lawlor, D.W.: Limitation to photosynthesis in water-stressed leaves: stomata vs. metabolism and the role of ATP. — Ann. Bot. 89: 871–885, 2002.
Liu, Y.B., Zhang, T.G., Li, X.R., Wang, G.: Protective mechanism of desiccation tolerance in Reaumuria Soongorica: Leaf abscission and sucrose accumulation in the stem. — Sci. China. Ser. C-Life Sci. 50: 15–21, 2007.
Lo Gullo, M.A., Salleo, S.: Different strategies of drought resistance in three Mediterranean scierophyllous trees growing in the same environmental conditions. — New Phytol. 108: 267–276, 1988.
Ma, C. C., Gao, Y. B., Guo, H. Y., Wang, J. L.: Photosynthesis, transpiration, and water use efficiency of Caragana microphylla, C. intermedia, and C. korshinskii. — Photosynthetica 42: 65–70, 2004.
Mahouachi, J., Socorro, A.R., Talon, M.: Responses of papaya seedling (Carica papaya L.) to water stress and re-hydration: growth, photosynthesis and mineral nutrient imbalance. — Plant Soil 281: 137–146, 2006.
Maroco, J.P., Pereira, J.S., Chaves, M.M.: Stomatal responses to leaf-to-air vapour pressure deficit in Sahelian species. — Aust. J. Plant Physiol. 24: 381–387, 1997.
McDowell, N., Pockman, W.T., Allen, C.D. et al..: Mechanisms of plant survival and morta-lity during drought: why do some plants survive while others succumb to drought? — New Phytol. 178: 719–739, 2008.
Morgan, P.W., Jordan, W.R., Davenport, T.L., Durham, J.I.: Abscission response to moisture stress, auxin transport inhibitors, and ethephon. — Plant Physiol. 59: 710–712, 1977.
Nilsen, E.T., Muller, W.H.: Phenology of the droughtdeciduous shrub Lotus scoparius: climatic controls and adaptive significance. — Ecol. Monogr. 51: 323–341, 1981.
Qian, Y.L., Fry, J.D., Upham, W.S.: Rooting and drought avoidance of warm-season turfgrasses and tall fescue in Kansas. — Crop Sci. 37: 699–704, 1997.
Schiller, P., Wolf, R., Hartung, W.: A scanning electron microscopical study of hydrated and dehydrated submerged leaves of the aquatic resurrection plant Chamaegigas intrepidus. — Flora 194: 97–102, 1999.
Sims, D.A., Gamon, J.A.: Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. — Remote Sens Environ. 81: 337–354, 2002.
Song, W.M., Zhou, H.Y., Jia, R. L., Zhao, X., Feng, L., Tan, H.J.: [Response of photosynthesis function and trehalose content of four desert plants to gradual drought stress.] — J. Desert Res. 28: 449–454, 2008. [In Chin.]
Tyree, M., Sperry, J.: Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Answers from a model. — Plant Physiol. 88: 574–580, 1988.
Varela, S.A., Gyenge, J.E., Fernández, M.E., Schlichter, T.: Seedling drought stress susceptibility in two deciduous Nothofagus species of NW Patagonia. — Trees 24: 443–453, 2010.
Wang, X.P., Li, X.R., Kang, E. S.: [Experiment on evapotranspiration of Xerophyte communities in a revegetated desert zone.] — J. Desert Res. 22: 363–367, 2002a. [In Chin.]
Wang, Y.R., Zeng, Y.J., Zhang, B. L.: [Water distribution patterns in different degraded desert grasslands of Reaumuria soongorica.] — Chin J. Appl. Ecol. 13: 962–966, 2002b. [In Chin.]
Xia, G.M., Kang, S.Z., Li, W.C., Wang, F., Qu, Y.P.: Diurnal and seasonal variation of stem sap flow for Caragana korshinskii on the arid desert region in Shiyang river basin of Gansu. — Acta Phytoecol Sin. 26: 1187–1193, 2006.
Xu, D.H., Bai, J., Li, J.H., Fang, X.W., Wang, G.: Changes of photosynthetic activity and carbohydrate content in resurrection plant Caragana korshinskii during de-hydration and rehydration. — Plant Stress 2: 45–49, 2008.
Xu, D.H., Su, P.X., Zhang, R.Y., Li, H.L., Zhao, L., Wang, G.: Photosynthetic parameters and carbon reserves of a resurrection plant Reaumuria soongorica during de-hydration and rehydration. — Plant Growth Regul. 60: 183–190, 2010.
Zhang, X.S.: Principles and optimal models for development of Maowusu sandy grassland. — Acta Phytoecol Sin. 18: 1–16, 1994.
Zhang, Z.S., Li, X.R., Liu, L.C., Jia, R.L., Zhang, J.G., Wang, T.: Distribution, biomass, and dynamics of roots in a revegetated stand of Caragana korshinskii in the Tengger Desert, northwestern China. — J. Plant Res. 122: 109–119, 2009.
Zheng, Y., Xie, Z., Gao, Y., Jiang, L., Shimizu, H., Tobe, K.: Germination responses of Caragana korshinskii Kom. to light, temperature and water stress. — Ecol. Res. 19: 553–558, 2004.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgments: This study was supported by the National Natural Sciences Foundation of China (No. 30900171 and No. 91025026) and China Postdoctoral Science Foundation (No. 20090450186).
Rights and permissions
About this article
Cite this article
Xu, D.H., Fang, X.W., Su, P.X. et al. Ecophysiological responses of Caragana korshinskii Kom. under extreme drought stress: Leaf abscission and stem survives. Photosynthetica 50, 541–548 (2012). https://doi.org/10.1007/s11099-012-0060-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-012-0060-4