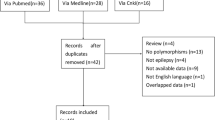
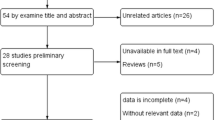
Abstract
BackgroundEPHX1 gene polymorphisms were recently acknowledged as an important source of individual variability in carbamazepine metabolism, but the result of that association still remains controversial. Aim of the review To obtain a more precise estimation of the associations between EPHX1 polymorphisms and carbamazepine metabolism and resistance. Methods The PubMed, EMBASE, Cochrane library, Chinese National Knowledge Infrastructure, Chinese Science and Technique Journals Database, China Biology Medicine disc and Wan fang Database were searched for appropriate studies regarding the rs1051740 and rs2234922 polymorphisms of EPHX1 up to September 2019. The meta-analysis was carried out using the Review Manager 5.3 software. The mean difference and 95% confidence interval were applied to assess the strength of the relationship. Results A total of 7 studies involving 1118 related epilepsy patients were included. EPHX1 rs1051740 polymorphism was significantly associated with adjusted concentrations of both carbamazepine (CC vs. TT: P = 0.02; CC vs. CT + TT: P = 0.005) and carbamazepine-10,11-epoxide (CC vs. CT + TT: P = 0.03). Furthermore, EPHX1 rs2234922 polymorphism was also observed to be significantly associated with decreased adjusted concentrations of carbamazepine-10,11-trans dihydrodiol (GG vs. GA + AA: P = 0.04) and CBZD:CBZE ratio (GG vs. AA: P = 0.008; GG vs. GA + AA: P = 0.0008). Nevertheless, the pooled analysis showed that the EPHX1 polymorphisms had no significant effect on CBZ resistance. Conclusion EPHX1 rs1051740 and rs2234922 polymorphisms may affect the carbamazepine metabolism; but carbamazepine resistance was not related to any of the single nucleotide polymorphisms investigated. These findings provided further evidence for individualized therapy of epilepsy patients in clinics.








Similar content being viewed by others
References
Brodie MJ, Dichter MA. Established antiepileptic drugs. Seizure. 1997;6:159–74.
Qu L, Fan Y, Wang W, Ma K, Yin Z. Development, validation and clinical application of an online-SPE-LC-HRMS/MS for simultaneous quantification of phenobarbital, phenytoin, carbamazepine, and its active metabolite carbamazepine 10,11-epoxide. Talanta. 2016;158:77–88.
Caruso A, Bellia C, Pivetti A, Agnello L, Bazza F, Scazzone C, et al. Effects of EPHX1 and CYP3A4 polymorphisms on carbamazepine metabolism in epileptic patients. Pharmgenomics Pers Med. 2014;7:117–20.
Tomalik-Scharte D, Lazar A, Fuhr U, Kirchheiner J. The clinical role of genetic polymorphisms in drug-metabolizing enzymes. Pharmacogenomics J. 2008;8:4–15.
Grover S, Gourie-Devi M, Baghel R, Sharma S, Bala K, Gupta M, et al. Genetic profile of patients with epilepsy on first-line antiepileptic drugs and potential directions for personalized treatment. Pharmacogenomics. 2010;11:927–41.
Ferraro TN, Buono RJ. The relationship between the pharmacology of antiepileptic drugs and human gene variation: an overview. Epilepsy Behav. 2005;7:18–36.
Kerr BM, Thummel KE, Wurden CJ, Klein SM, Kroetz DL, Gonzalez FJ, et al. Human liver carbamazepine metabolism Role of CYP3A4 and CYP2C8 in 10,11-epoxide formation. Biochem Pharmacol. 1994;47:1969–79.
Pearce RE, Vakkalagadda GR, Leeder JS. Pathways of carbamazepine bioactivation in vitro I Characterization of human cytochromes P450 responsible for the formation of 2- and 3-hydroxylated metabolites. Drug Metab Dispos. 2002;30:1170–9.
Thorn CF, Leckband SG, Kelsoe J, Leeder JS, Muller DJ, Klein TE, et al. PharmGKB summary: carbamazepine pathway. Pharmacogenet Genomics. 2011;21:906–10.
Makmor-Bakry M, Sills GJ, Hitiris N, Butler E, Wilson EA, Brodie MJ. Genetic variants in microsomal epoxide hydrolase influence carbamazepine dosing. Clin Neuropharmacol. 2009;32:205–12.
Patsalos PN, Berry DJ, Bourgeois BF, Cloyd JC, Glauser TA, Johannessen SI, et al. Antiepileptic drugs–best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring. ILAE Comm Ther Strateg Epilepsia. 2008;49:1239–76.
Kwan P, Brodie MJ. Effectiveness of first antiepileptic drug. Epilepsia. 2001;42:1255–60.
Nakajima Y, Saito Y, Shiseki K, Fukushima-Uesaka H, Hasegawa R, Ozawa S, et al. Haplotype structures of EPHX1 and their effects on the metabolism of carbamazepine-10,11-epoxide in Japanese epileptic patients. Eur J Clin Pharmacol. 2005;61:25–34.
Loscher W, Klotz U, Zimprich F, Schmidt D. The clinical impact of pharmacogenetics on the treatment of epilepsy. Epilepsia. 2009;50:1–23.
Vaclavikova R, Hughes DJ, Soucek P. Microsomal epoxide hydrolase 1 (EPHX1): gene, structure, function, and role in human disease. Gene. 2015;571:1–8.
Zhu QS, Qian B, Levy D. Regulation of human microsomal epoxide hydrolase gene (EPHX1) expression by the transcription factor GATA-4. Biochim Biophys Acta. 2004;1676:251–60.
Hassett C, Aicher L, Sidhu JS, Omiecinski CJ. Human microsomal epoxide hydrolase: genetic polymorphism and functional expression in vitro of amino acid variants. Hum Mol Genet. 1994;3:421–8.
Hassett C, Lin J, Carty CL, Laurenzana EM, Omiecinski CJ. Human hepatic microsomal epoxide hydrolase: comparative analysis of polymorphic expression. Arch Biochem Biophys. 1997;337:275–83.
Smith CA, Harrison DJ. Association between polymorphism in gene for microsomal epoxide hydrolase and susceptibility to emphysema. Lancet. 1997;350:630–3.
Kitteringham NR, Davis C, Howard N, Pirmohamed M, Park BK. Interindividual and interspecies variation in hepatic microsomal epoxide hydrolase activity: studies with cis-stilbene oxide, carbamazepine 10, 11-epoxide and naphthalene. J Pharmacol Exp Ther. 1996;278:1018–27.
Ma CL, Jiao Z, Wu XY, Hong Z, Wu ZY, Zhong MK. Association between PK/PD-involved gene polymorphisms and carbamazepine-individualized therapy. Pharmacogenomics. 2015;16:1499–512.
Hung CC, Chang WL, Ho JL, Tai JJ, Hsieh TJ, Huang HC, et al. Association of polymorphisms in EPHX1, UGT2B7, ABCB1, ABCC2, SCN1A and SCN2A genes with carbamazepine therapy optimization. Pharmacogenomics. 2012;13:159–69.
Chbili C, Fathallah N, Laouani A, Nouira M, Hassine A, Ben Amor S, et al. Effects of EPHX1 and CYP3A4*22 genetic polymorphisms on carbamazepine metabolism and drug response among Tunisian epileptic patients. J Neurogenet. 2016;30:16–211.
Zhu X, Yun W, Sun X, Qiu F, Zhao L, Guo Y. Effects of major transporter and metabolizing enzyme gene polymorphisms on carbamazepine metabolism in Chinese patients with epilepsy. Pharmacogenomics. 2014;15:1867–79.
Ru JL. Analysis of monitoring results of carbamazepine serum concentrations and association with ABCB1, EPHX1 genetic polymorphisms. Shengyang: Chinese Medical University; 2012.
Daci A, Beretta G, Vllasaliu D, Shala A, Govori V, Norata GD, et al. Polymorphic Variants of SCN1A and EPHX1 Influence Plasma Carbamazepine Concentration, Metabolism and Pharmacoresistance in a Population of Kosovar Albanian Epileptic Patients. PLoS ONE. 2015;10:e0142408.
Puranik YG, Birnbaum AK, Marino SE, Ahmed G, Cloyd JC, Remmel RP, et al. Association of carbamazepine major metabolism and transport pathway gene polymorphisms and pharmacokinetics in patients with epilepsy. Pharmacogenomics. 2013;14:35–45.
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–77.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. The Ottawa Health Research Institute. 2013. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 3 July 2019.
Yun W, Zhang F, Hu C, Luo X, Xue P, Wang J, et al. Effects of EPHX1, SCN1A and CYP3A4 genetic polymorphisms on plasma carbamazepine concentrations and pharmacoresistance in Chinese patients with epilepsy. Epilepsy Res. 2013;107:231–7.
Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–85.
Yadegary MA, Maemodan FG, Nayeri ND, Ghanjekhanlo A. The effect of self-management training on health-related quality of life in patients with epilepsy. Epilepsy Behav. 2015;50:108–12.
Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46:470–2.
Leite CE, Petersen GO, Lunardelli A, Thiesen FV. A high-performance liquid chromatography method for the determination of carbamazepine and carbamazepine-10,11-epoxide and its comparison with chemiluminescent immunoassay. Clin Chem Lab Med. 2009;47:458–63.
Shen S, Elin RJ, Soldin SJ. Characterization of cross reactivity by carbamazepine 10,11-epoxide with carbamazepine assays. Clin Biochem. 2001;34:157–8.
Tate SK, Depondt C, Sisodiya SM, Cavalleri GL, Schorge S, Soranzo N, et al. Genetic predictors of the maximum doses patients receive during clinical use of the anti-epileptic drugs carbamazepine and phenytoin. Proc Natl Acad Sci USA. 2005;102:5507–12.
Naidoo P, Naidoo RN, Ramkaran P, Asharam K, Chuturgoon AA. The Tyr113His T/C rs1051740 and 'very slow' phenotype of the EPHX1 gene alters miR-26b-5p and miR-1207-5p expression in pregnancy. Gene. 2017;633:71–81.
Benhamou S, Reinikainen M, Bouchardy C, Dayer P, Hirvonen A. Association between lung cancer and microsomal epoxide hydrolase genotypes. Cancer Res. 1998;58:5291–3.
Raubenheimer PJ, Levitt NS. A case of generalised allergic reaction to human insulin. J Endocrinol Metab Diabetes S Afr. 2004;9:18–20.
Tuanthaisong K, Chinvarun Y, Tantisira MH, Kijsanayotin P. Influence of EPHX1 polymorphism and clinical factors on carbamazepine resistant epilepsy. J Pharm Sci. 2012;36:24–9.
Lv Y, Zheng X, Shi M, Wang Z, Cui L. Different EPHX1 methylation levels in promoter area between carbamazepine-resistant epilepsy group and carbamazepine-sensitive epilepsy group in Chinese population. BMC Neurol. 2019;19:114.
Funding
This work was supported by Grants from the National Science Foundation of China (Nos. 81460560 and 81960664) and the Applied Basic Research Program of Yunnan Province of China (No. 2017FB134).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Forest plot for association between EPHX1 rs1051740 polymorphism and CBZ metabolism in heterozygous model (a). Sensitivity analysis for effect of EPHX1 rs1051740 polymorphism on CDRCBZ in heterozygous model (b). (TIFF 29906 kb)
Fig. S2
Forest plot for association between EPHX1 rs1051740 polymorphism and CBZ metabolism in dominant model (a). Sensitivity analysis for effect of EPHX1 rs1051740 polymorphism on CDRCBZ, CDRCBZE and CBZE:CBZ ratio in dominant model (b). (TIFF 36145 kb)
Fig. S3
Forest plot for association between EPHX1 rs1051740 polymorphism and CBZ metabolism in co-dominant model. (TIFF 11389 kb)
Fig. S4
Forest plot for association between EPHX1 rs2234922 polymorphism and CBZ metabolism in heterozygous model (a). Sensitivity analysis for effect of EPHX1 rs2234922 polymorphism on CDRCBZ, CDRCBZE, CDRCBZD and CBZD:CBZE ratio in heterozygous model (b). (TIFF 37913 kb)
Fig. S5
Forest plot for association between EPHX1 rs2234922 polymorphism and CBZ metabolism in dominant model (a). Sensitivity analysis for effect of EPHX1 rs2234922 polymorphism on CDRCBZ, CDRCBZE, and CBZD:CBZE ratio in dominant model (b). (TIFF 34796 kb)
Fig. S6
Forest plot for association between EPHX1 rs2234922 polymorphism and CBZ metabolism in co-dominant model (a). Sensitivity analysis for effect of EPHX1 rs2234922 polymorphism on CDRCBZ, CDRCBZE, CDRCBZD and CBZD:CBZE ratio in co-dominant model (b). (TIFF 37915 kb)
Rights and permissions
About this article
Cite this article
Zhao, GX., Shen, ML., Zhang, Z. et al. Association between EPHX1 polymorphisms and carbamazepine metabolism in epilepsy: a meta-analysis. Int J Clin Pharm 41, 1414–1428 (2019). https://doi.org/10.1007/s11096-019-00919-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-019-00919-y