Abstract
Background Data are lacking about the extent of drug-related problems in hospitalized patients with COPD in China. Objective Identify types and causes of drug-related problems and assess interventions performed by pharmacists. Setting Study was conducted in an academic teaching hospital in Shanghai, China. Method Between June 2017 and July 2018, 393 patients admitted to hospital for acute exacerbation of COPD hospitalized were enrolled. Patient demographics and clinical characteristics were collected. The drug-related problems and interventions were recorded and analyzed based on the Pharmaceutical Care Network Europe (PCNE)-DRP V 8.02 classification. Main outcome measures The number, types, causes, interventions, and outcomes of the problems were analyzed. Results A total of 640 DRPs, with 763 corresponding causes, were identified for 393 patients. “Treatment safety P2” was the most common type of problem (54.2%; 347/640), and the most common causes were “drug selection C1” (24.2%; 185/763), “dose selection C3” (21.5%; 164/763) and “treatment duration C4” (17.7%; 135/763). Antibiotics, corticosteroids, and proton pump inhibitors were the three primary medication classes associated with DRPs. Patients, hospitalized for more than eight days, taking ten or more drugs or having renal dysfunctions were more likely to have drug-related problems. Pharmacists totally proposed 1557 interventions to address the problems. Most interventions (91.0%; 1418/1557) were accepted, and 91.6% of the problems were solved. Conclusion The prevalence of drug-related problems among the studied COPD patients was high. Pharmacists can have an important role in addressing the problems and optimizing the safety and effectiveness of therapies for hospitalized COPD patients.
Similar content being viewed by others
Impacts on practice
-
Drug-related problems (DRPs) are common in hospitalized patients with chronic obstructive pulmonary disease (COPD) in China.
-
Pharmacists may play an important role in identifying and solving DRPs in hospitalized COPD patients.
-
COPD patients, with renal impairment, hospitalized for more than eight days and taking ten or more drugs are likely to have an increased number of DRPs, requiring prioritized pharmaceutical care.
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide [1], acute exacerbations of COPD are common events that often lead to hospitalization with worsened quality of life, increased health-care costs, and increased mortality [2]. In China, COPD accounts for 1.6% of all hospital admissions [3]. The hospital represents a unique setting. Most hospitalized COPD patients are elderly patients (≥ 70 years) [4] and burdened with co-morbidities such as cardiovascular diseases, diabetes, and hypertension [5, 6]. Because of these compounding patient factors, patients hospitalized for COPD are at a higher risk of experiencing drug-related problems (DRPs). Various classification systems have been used to categorize DRPs, and the Pharmaceutical Care Network Europe (PCNE) Classification is a validated system in hospital settings [7, 8].
Most studies about DRPs among COPD patients are conducted in community patients with stable COPD [9,10,11,12], while the extent and characteristics of DRPs in hospitalized acute exacerbation COPD patients are unknown. Treatment regimens for stable and acute exacerbation COPD patients are different [13]. Hospitalized patients may possess a higher risk of DRPs [14], therefore a better knowledge of DRPs among hospitalized acute exacerbation COPD patients is needed.
Aim of the study
The primary objective was to categorize DRPs identified in hospitalized COPD patients and to assess interventions provided by pharmacists. The secondary objective was to identify factors associated with DRPs.
Ethics approval
The study was approved by the Ethical Committee of Tongren Hospital, Shanghai Jiao Tong University School of Medicine (Shanghai Tongren Hospital Ethics Committee 2016-021-01). Both the objectives and methodology of the study were explained to patients, and written consent forms were obtained from study participants.
Methods
Study design, setting, and participants
Data on DRPs in hospitalized COPD patients were prospectively collected from June 2017 to July 2018. Study subjects were COPD patients admitted at the Department of Respiratory Medicine or Respiratory Intensive Care Unit at the Tongren Hospital, Shanghai Jiao Tong University, a 1500-bed teaching hospital in Shanghai, the largest city in China.
Inclusion criteria were (1) patients with a confirmed diagnosis of COPD per the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines [13]; and (2) patients admitted to hospital for acute exacerbation of COPD. Exclusion criteria were (1) patients in terminal or palliative care [15], or (2) patients unable to understand the consent form written in Chinese.
Data collection
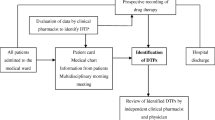
Two qualified doctors from the respiratory units were trained to assist in data collection. Five clinical pharmacists, had at least five-year hospital pharmacy experiences and have completed the PCNE-DRP classification training, attended COPD medication management sessions. Patient demographics and clinical information (COPD condition, co-morbidities, and treatment regimens) were obtained from medical records and patient interviews. Treatment regimens were assessed for indication, effectiveness, dosage, directions, practicability, drug–drug interactions, contraindications, duplication, duration, side effects, compliance, untreated indication, and monitoring information. The duration of individual patient data ranged from hospital admission to discharge.
PCNE-DRP classification
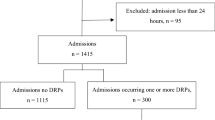
Clinical guidelines [1] and drug databases were consulted to identify DRPs and propose interventions. The Screening Tool of Older Persons’ Prescriptions (STOPP) and Screening Tool to Alert doctors to Right Treatment (START) tool kits were used to identify potentially inappropriate medicines [16]. DRPs were classified according to the PCNE-DRP V8.02 classification system. One Problem (P) may have multiple Causes (C), and lead to more than one Interventions (I), but it leads to only one Outcome (O). Data were independently verified by three pharmacists as shown in Fig. 1. One clinical pharmacist investigated and categorized DRPs based on the patient’s clinical information, and a second pharmacist re-investigated and re-classified those DRPs to ensure consistency. When inconsistency existed, a third pharmacist would re-investigate and re-classify. If results remained to be inconsistent, a consensus was reached through discussion [17].
Statistical analysis
Individual and problem-level analyses were performed separately because one patient may have multiple DRPs. All statistical analyses were carried out using SPSS (Version 23.0). Both univariate and multivariate analyses were conducted. Based on the univariate analysis, variables that were significant (P < 0.05) were included in the multivariate analysis to control for confounders and to identify factors independently associated with the number of DRPs. The results of univariate andmultivariate analysis were reported as crude odds ratio (COR) and adjusted odds ratio (AOR) at 95% confidence intervals (95% CI), respectively. A P value of < 0.05 was considered significant.
Results
Patient characteristics
As shown in Fig. 1, a total of 428 hospitalized patients with COPD were initially recruited into the study. A total of 35 patients withdrew during the assessment period. Data was ultimately collected and analyzed from 393 patients. The mean age was 76.4 ± 12.3 years, 77.6% were males, and 93.9% were past or current smokers (52 never smoke). The average FEV1 was 42.4%, rated as severe COPD based on the 2017 GOLD guideline. Based on stratification according to symptoms and prior history of exacerbations, 66.9% (263/393) of patients were categorized as Group D and 24.7% (97/393) were categorized as Group C COPD patients. Together these two groups accounted for 91.6% of total study patients. Participants had an average 4.2 co-morbidities, with 89.6% (352/393) of patients having hypertension, 53.2% (209/393) with coronary artery disease, and 49.4% with diabetes (194/393). The details of the patients’ demographics and clinical characteristics are listed in Table 1. Polypharmacy (taking five or more medications) [18] was common, accounting for 96.9% (381/393) of patients, with an average of 11.2 medications per patient. The five most frequent medication classes were antibiotics, anti-hypertensives, bronchodilators,corticosteroids, and expectorants.
Drug-related problems identified for inpatients with COPD
A total of 640 DRPs were identified, averaging 1.6 per patient, with more than half (56.7%; 223/393) of the patients having at least one DRPs. The distribution and causes of DRPs are shown in Fig. 2 and Table 2. Among the 640 DRPs, “treatment safety P2” was the major type of DRP (54.2%; 347/640) identified, followed by “treatment effectiveness P1” (24.1%; 154/640). A total of 763 causes were categorized, with the three most frequent causes being “drug selection C1” (24.2%; 185/763), “dose selection C3” (21.5%; 164/763) and “treatment duration C4” (17.7%; 135/763). Pharmacists proposed 1557 interventions to solve the DRPs, averaging 2.4 interventions per DRP identified. As shown in Fig. 3, about half of the interventions were made at the “drug level I3” (45.5%; 708/1557) followed by at the “prescriber level I1” (32.6%; 507/1557) and at the “patient level I2” (16.2%; 252/1557). As shown in Table 3, the top three major drug classes causing DRPs were antibiotics (36.7%; 235/640), corticosteroids (19.8%; 127/640), and proton pump inhibitors (PPIs; 10.2%; 65/640).
Analysis of factors associated with the number of drug-related problems
Univariate binary logistic regression analysis showed that patients who had three or more disease conditions, took ten or more drugs, stayed in hospital eight or more days and renal dysfunction were more likely to have DRPs. Multivariate logistic regression analysis indicated that only ten or more drugs taken, eight or more days stayed in hospital and renal dysfunction had a significant association with DRPs. The details are summarized in Table 4.
Acceptance of interventions and the status of drug-related problems
Total 91.0% interventions (1418/1557) were accepted. Among these, 80.0% were fully implemented. Most identified DRPs (91.6%; 586/640) were solved (Table 5).
Discussion
To the best of our knowledge, this is the first prospective study conducted in China to categorically evaluate DRPs in hospitalized COPD patients, and to analyze the utility of pharmacist intervention. Remarkably, more than half (56.7%) of the study participants had at least one DRP, with an average of 1.6 DRPs per patient. But this finding is consistent with a recent study in community-dwelling COPD adults using the PCNE classification [19], further suggesting that hospitalized COPD patients were also at high risk for DRPs.
The most commonly identified DRP type was “treatment safety P2”. However, this was inconsistent with the findings of the previous study on COPD in which the “treatment effectiveness P1” was the most common DRP category [19]. Discrepancy in the type of DRP may be explained by the following arguments: (1) the previous study was in the community setting, while the current study was in the hospital setting. Antibiotics and corticosteroids were more often used for the treatment of acute COPD exacerbations in hospitalized patients [20], thereby being associated with more drug-safety problems than community COPD patients [21]; (2) more drugs were administered to hospitalized patients, which required clinical pharmacists to pay more attention on drug regimens to improve drug safety [22], such as long-acting loop diuretic using as blood pressure-lowering agents in diabetic hypertension patients identified as potentially inappropriate medicines by the START tool; and (3) different PCNE-DRP classification versions were used. This study used the PCNE-DRP V8.2 classification system while the previous study used the V6.2 version. These two versions are not compatible as more sections were added or revised in V8.2. The three major sub-categories of DRP causes were “drug dose too high C3.2”, “duration of treatment too long C4.2”, and “patient administers/uses the drug in a wrong way C7.8”. This indicates that the pharmacist should provide necessary patient education on the correct use of drugs when providing medication review.
Antibiotics, corticosteroids, and PPIs were the top three DRP-causing drug classes identified in our study. Antibiotics and corticosteroids are first-line treatments for patients with COPD exacerbation [23]. As further confirmed through our study, the over-prescribing of antibiotics in China is particularly rampant. The imprudent prescribing of antibiotics has numerous unintended consequences. DRPs associated with antibiotics are commonly related to antibiotic selection, dose, and duration. Examples of common antibiotic DRPs include inappropriate renal dose adjustment [24] and lengthy durations of treatment. In our study, pharmacists provided interventions aimed at improving the use of antibiotics by suggesting “drug change I3.1” or “dosage change I 3.2” through participation in clinical rounds, consultations, and case discussions. When treating hospitalized COPD patients, providers and pharmacists should consider the effect of renal function on the pharmacodynamics and pharmacokinetics of drugs to avoid the occurrence of DRPs [25, 26], and pharmacists play an important role in the rational use of antibiotics given their specialized training in pharmacotherapy [27].
The two main causes of DRPs regarding corticosteroid use were “patient uses the drug in a wrong way C7.8” and “inappropriate outcome monitoring C8.1”. In this study, pharmacists found that about 60% of patients were using their inhaler devices incorrectly although they were provided with instruction leaflets during prescription dispensing at their outpatient pharmacy. For many patients, proper use of inhalers is difficult. Studies have demonstrated that many patients fail to correctly use their inhalers even when written instructions are provided, therefore verbal instructions and device demonstration given by pharmacists are necessary during medication education [19]. In our study, once incorrect technique was discovered, patients were informed that the correct technique was essential for COPD control, and a “teach-back” technique was applied to demonstrate and teach the correct use of inhalers. In mainland China, the role of the clinical pharmacist is still in early development, and only major academic teaching hospitals have clinical pharmacy services available for hospitalized patients [28]. As demonstrated in this study inadequate patient education from healthcare professionals is common suggesting that pharmacists are urgently needed in COPD care management, given their medication-related expertise [19, 29]. Lack of or inappropriate safety monitoring, such as diabetic patients without monitoring for hyperglycemia upon commencement of systemic high-dose steroid therapy for acute COPD exacerbations [30], was another major corticosteroid-related problem. Over 90% of patients in this study had hypertension and/or diabetes, and constant monitoring of both blood pressure and blood glucose levels was crucial in order to prevent the occurrence of potential problems [30]. Pharmacists should play an important role in monitoring treatment efficacy and safety.
Inappropriate combinations of drugs with PPI were the major PPI-related DRPs, such as the combined use of clopidogrel and omeprazole. This interaction may increase the incidence of major adverse cardiovascular events especially in patients with coronary artery disease [31]. The high prevalence of PPI-related DRPs highlights the need for pharmacists to comprehensively collect and assess patients’ medical histories and medication regimen through patients’ interviews, in order to detect DRPs that may otherwise be missed with just relying on medical chart review.
In our study, age and gender were not factors predicting the occurrence of DRPs. However, in multivariate logistic regression, factors of hospitalized for more than eight days, taking ten or more drugs or renal dysfunctions were found to be independent predictors of DRPs. This is a natural consequence of using multiple medications (owing to longer hospitalizations) and therapeutic classes (owing to multi co morbidities) simultaneously.
The high acceptance rate of pharmacist-proposed interventions by prescribers and patients demonstrated a strong trust between pharmacists, physicians, and patients, and this highlights the role of clinical pharmacists in the team-based care in COPD patients. The following are potential impacts of our study: (1) clinical pharmacist participation in COPD care is important in identifying, solving, and optimizing the pharmacotherapy of this vulnerable patient population; (2) face-to-face inhaler demonstration is needed to teach patients on the correct use of inhalers; and (3) our COPD pharmaceutical care model could be adopted by other hospitals in China to provide better COPD management in China. This study has the following limitations: (1) this is a single center study with a relatively small sample size, and the results may not generalizable to other hospitals; and (2) only DRP was used as a clinical outcome indicator, other clinical outcome indications were not assessed.
Conclusion
Our study demonstrates that within China, drug-related problems are remarkably high in hospitalized patients with COPD. More than half (56.7%) of the participants in this study had at least one DRP, with an average of 1.6 DRPs per patient. This study not only categorizes DRPs in COPD patients for the first time, but also demonstrates the value of clinical pharmacy service in the care of COPD patients.
References
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):1700214.
Marchetti N, Criner GJ, Albert RK. Preventing acute exacerbations and hospital admissions in COPD. Chest. 2013;143(5):1444–54.
Fang X, Wang X, Bai C. COPD in China: the burden and importance of proper management. Chest. 2011;139(4):920–9.
Yin P, Wang H, Vos T, Li Y, Liu S, Liu Y, et al. A subnational analysis of mortality and prevalence of COPD in China from 1990 to 2013: findings from the global burden of disease study 2013. Chest. 2016;150(6):1269–80.
Mannino DM, Higuchi K, Yu TC, Zhou H, Li Y, Tian H, et al. Economic burden of COPD in the presence of comorbidities. Chest. 2015;148(1):138–50.
Steveling EH, Clarenbach CF, Miedinger D, Enz C, Durr S, Maier S, et al. Predictors of the overlap syndrome and its association with comorbidities in patients with chronic obstructive pulmonary disease. Respiration. 2014;88(6):451–7.
Lampert ML, Kraehenbuehl S, Hug BL. Drug-related problems: evaluation of a classification system in the daily practice of a Swiss University Hospital. Pharm World Sci. 2008;30(6):768–76.
Nielsen TR, Andersen SE, Rasmussen M, Honore PH. Clinical pharmacist service in the acute ward. Int J Clin Pharm. 2013;35(6):1137–51.
Altowaijri A, Phillips CJ, Fitzsimmons D. A systematic review of the clinical and economic effectiveness of clinical pharmacist intervention in secondary prevention of cardiovascular disease. J Manag Care Pharm. 2013;19(5):408–16.
Jarab AS, Alqudah SG, Khdour M, Shamssain M, Mukattash TL. Impact of pharmaceutical care on health outcomes in patients with COPD. Int J Clin Pharm. 2012;34(1):53–62.
Ottenbros S, Teichert M, de Groot R, Griens F, Sodihardjo F, Wensing M, et al. Pharmacist-led intervention study to improve drug therapy in asthma and COPD patients. Int J Clin Pharm. 2014;36(2):336–44.
Zhong H, Ni XJ, Cui M, Liu XY. Evaluation of pharmacist care for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J Clin Pharm. 2014;36(6):1230–40.
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–82.
Pourrat X, Roux C, Bouzige B, Garnier V, Develay A, Allenet B, et al. Impact of drug reconciliation at discharge and communication between hospital and community pharmacists on drug-related problems: study protocol for a randomized controlled trial. Trials. 2014;15:260.
Mechler K, Liantonio J. Palliative care approach to chronic diseases: end stages of heart failure, chronic obstructive pulmonary disease, liver failure, and renal failure. Prim Care. 2019;46(3):415–32.
Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (screening tool of older person’s prescriptions) and START (screening tool to alert doctors to right treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83.
Hsu WT, Shen LJ, Lee CM. Drug-related problems vary with medication category and treatment duration in Taiwanese heart failure outpatients receiving case management. J Formos Med Assoc. 2016;115(5):335–42.
Hoffmann F, Schmiemann G, Dorks M. Assessment of polypharmacy: a question of definition and underlying data. Z Evid Fortbild Qual Gesundhwes. 2016;113:27–35.
Apikoglu-Rabus S, Yesilyaprak G, Izzettin FV. Drug-related problems and pharmacist interventions in a cohort of patients with asthma and chronic obstructive pulmonary disease. Respir Med. 2016;120:109–15.
Laue J, Reierth E, Melbye H. When should acute exacerbations of COPD be treated with systemic corticosteroids and antibiotics in primary care: a systematic review of current COPD guidelines. NPJ Prim Care Respir Med. 2015;25:15002.
Suggett E, Marriott J. Risk factors associated with the requirement for pharmaceutical intervention in the hospital setting: a systematic review of the literature. Drugs Real World Outcomes. 2016;3(3):241–63.
Schorr SG, Eickhoff C, Feldt S, Hohmann C, Schulz M. Exploring the potential impact of hospital ward-based pharmacy interns on drug safety. Pharmazie. 2014;69(4):316–20.
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Respirology. 2017;22(3):575–601.
Silva C, Ramalho C, Luz I, Monteiro J, Fresco P. Drug-related problems in institutionalized, polymedicated elderly patients: opportunities for pharmacist intervention. Int J Clin Pharm. 2015;37(2):327–34.
Lenssen R, Heidenreich A, Schulz JB, Trautwein C, Fitzner C, Jaehde U, et al. Analysis of drug-related problems in three departments of a German University hospital. Int J Clin Pharm. 2016;38(1):119–26.
Abunahlah N, Elawaisi A, Velibeyoglu FM, Sancar M. Drug related problems identified by clinical pharmacist at the Internal Medicine Ward in Turkey. Int J Clin Pharm. 2018;40(2):360–7.
Zhu M, Guo DH, Liu GY, Pei F, Wang B, Wang DX, et al. Exploration of clinical pharmacist management system and working model in China. Pharm World Sci. 2010;32(4):411–5.
Yang J, Meng L, Liu Y, Lv L, Sun S, Long R, et al. Drug-related problems among community-dwelling older adults in mainland China. Int J Clin Pharm. 2018;40(2):368–75.
Tommelein E, Mehuys E, Van Hees T, Adriaens E, Van Bortel L, Christiaens T, et al. Effectiveness of pharmaceutical care for patients with chronic obstructive pulmonary disease (PHARMACOP): a randomized controlled trial. Br J Clin Pharmacol. 2014;77(5):756–66.
Fong AC, Cheung NW. The high incidence of steroid-induced hyperglycaemia in hospital. Diabetes Res Clin Pract. 2013;99(3):277–80.
Huang B, Huang Y, Li Y, Yao H, Jing X, Huang H, et al. Adverse cardiovascular effects of concomitant use of proton pump inhibitors and clopidogrel in patients with coronary artery disease: a systematic review and meta-analysis. Arch Med Res. 2012;43(3):212–24.
Acknowledgements
The authors would like to thank all the patients that agreed to participate in this study. We also thank Dr.’s Lucas Sheehan and HaiAn Zheng at Albany College of Pharmacy and Health Sciences for collaborative discussion and manuscript review.
Funding
This study were supported by a grant from the 2014 Changning district health and family planning system medical specialty project (2014201001) and 2018 Scientific Research Topics of Shanghai Health and Family Planning Commission (201840079).
Conflicts of interest
All authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Li, Q., Qu, H.J., Lv, D. et al. Drug-related problems among hospitalized patients with COPD in mainland China. Int J Clin Pharm 41, 1507–1515 (2019). https://doi.org/10.1007/s11096-019-00913-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-019-00913-4