Abstract
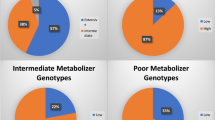
BackgroundCYP2C19 loss-of-function polymorphic alleles (*2 and *3) have been documented to impair clopidogrel metabolism, and represent a risk factor for major adverse cardiac events. CYP2C19 polymorphism exhibits marked ethnic heterogeneity. Objective To determine the prevalence of CYP2C19 *2 and *3 alleles in a cohort of Palestinian patients managed with percutaneous coronary intervention and dual antiplatelet therapy, and to determine their role in causing major adverse cardiac events. Setting The blood samples were collected at the European Gaza Hospital, and the molecular techniques performed at the molecular genetics laboratory of the Islamic university of Gaza. Method The frequency of CYP2C19 *2 and *3 alleles was determined in 110 patients managed with percutaneous coronary intervention and clopidogrel. Genotyping was performed by PCR–RFLP. Personal and clinical data was obtained from patient record and 6-month follow-up for major adverse cardiac events. Main outcome measureCYP2C19 genotype, personal and clinical data and incidence of major adverse cardiac events. Results The frequency of CYP2C19 *1, *2 and *3 alleles was 82.3%, 15.5% and 2.3% respectively. Genotyping analysis showed that, 67.3% were homozygotes for CYP2C19 *1, 27.3% were *1/*2, 2.7% with *1/*3 genotype, 1.8% were *2/*3 and 0.9% were *2/*2. These frequencies were consistent with those of Caucasian populations. According to this study the poor metabolizers phenotype frequency was 2.7%, which is in the same range reported in Caucasians (2–5%) and lower than Oriental populations 13–23%. A strong significant relation was found between major adverse cardiac events and carrying the variant allele CYP2C19 *2 (P = 0.001). On the other hand, there was no significant relation between major adverse cardiac events and carrying the variant allele CYP2C19 *3 (P = 0.324). Conclusion The CYP2C19 *2 allele is relatively common in our population, and its associated reduced metabolic activity deserves attention as it leads to an increased incidence of major adverse cardiac events in the follow-up of patients receiving clopidogrel.
Similar content being viewed by others
References
WHO. Cardiovascular diseases (CVDs). In: Fact sheets. WHO. 2017. http://www.who.int/mediacentre/factsheets/fs317/en/. Accessed 19 Oct 2018.
Bowry AD, Lewey J, Dugani SB, Choudhry NK. The burden of cardiovascular disease in low- and middle-income countries: epidemiology and management. Can J Cardiol. 2015;31(9):1151–9.
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–603.
Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35(37):2541–619.
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: an Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2016;133(11):1135–47.
Ge J, Yu H, Li J. Acute coronary stent thrombosis in modern era: etiology, treatment, and prognosis. Cardiology. 2017;137(4):246–55.
Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134(10):e123–55.
Steinhubl SR, Berger PB, Mann JT III, Fry ET, DeLago A, Wilmer C, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288(19):2411–20.
Savcic M, Hauert J, Bachmann F, Wyld PJ, Geudelin B, Cariou R. Clopidogrel loading dose regimens: kinetic profile of pharmacodynamic response in healthy subjects. Semin Thromb Hemost. 1999;25 Suppl 2:15–9.
Nurden PSPNA, Herbert SL-TJ-M. Clopidogrel: a review of its mechanism of action. Platelets. 1998;9(3–4):251–5.
Savi P, Herbert JM, Pflieger AM, Dol F, Delebassee D, Combalbert J, et al. Importance of hepatic metabolism in the antiaggregating activity of the thienopyridine clopidogrel. Biochem Pharmacol. 1992;44(3):527–32.
Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, et al. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost. 2000;84(5):891–6.
Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38(1):92–9.
Ray S. Clopidogrel resistance: the way forward. Indian Heart J. 2014;66(5):530–4.
Gurbel PA, Tantry US. Clopidogrel resistance? Thromb Res. 2007;120(3):311–21.
Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360(4):354–62.
Sofi F, Giusti B, Marcucci R, Gori AM, Abbate R, Gensini GF. Cytochrome P450 2C19* 2 polymorphism and cardiovascular recurrences in patients taking clopidogrel: a meta-analysis. Pharmacogenomics J. 2011;11(3):199–206.
Subraja K, Dkhar S, Priyadharsini R, Ravindra B, Shewade D, Satheesh S, et al. Genetic polymorphisms of CYP2C19 influences the response to clopidogrel in ischemic heart disease patients in the South Indian Tamilian population. Eur J Clin Pharmacol. 2013;69(3):415–22.
Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304(16):1821–30.
Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA. 2011;306(24):2704–14.
Kubica A, Kozinski M, Grzesk G, Fabiszak T, Navarese EP, Goch A. Genetic determinants of platelet response to clopidogrel. J Thromb Thrombolysis. 2011;32(4):459.
Collet J-P, Hulot J-S, Pena A, Villard E, Esteve J-B, Silvain J, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. The Lancet. 2009;373(9660):309–17.
Wu H, Qian J, Xu J, Sun A, Sun W, Wang Q, et al. Effects of CYP2C19 variant alleles on postclopidogrel platelet reactivity and clinical outcomes in an actual clinical setting in China. Pharmacogenetics Genomics. 2012;22(12):887–90.
Jeong YH, Abadilla KA, Tantry US, Park Y, Koh JS, Kwak CH, et al. Influence of CYP2C19*2 and *3 loss-of-function alleles on the pharmacodynamic effects of standard- and high-dose clopidogrel in East Asians undergoing percutaneous coronary intervention: the results of the ACCEL-DOUBLE-2N3 study. J Thromb Haemost. 2013;11(6):1194–7.
Mejin M, Tiong WN, Lai LY, Tiong LL, Bujang AM, Hwang SS, et al. CYP2C19 genotypes and their impact on clopidogrel responsiveness in percutaneous coronary intervention. Int J Clin Pharm. 2013;35(4):621–8.
Ibeanu GC, Blaisdell J, Ghanayem BI, Beyeler C, Benhamou S, Bouchardy C, et al. An additional defective allele, CYP2C19* 5, contributes to the S-mephenytoin poor metabolizer phenotype in Caucasians. Pharmacogenetics Genomics. 1998;8(2):129–36.
Dean L. Clopidogrel Therapy and CYP2C19 Genotype. In: Pratt V, McLeod H, Rubinstein W, Dean L, Kattman B, Malheiro A, editors. Medical Genetics Summaries [Internet]. Bethesda, MD: National Center for Biotechnology Information; 2012.
Palestinian Health Information Center. Deaths in Gaza strip. In: Madi K, editor. 2016 Annual Report. Gaza, Palestine: Ministry of health; 2017.
Palestinian Health Information Center. Hospitals in Gaza Strip. In: Nassar B, editor. 2016 Annual Report. Gaza, Palestine: Ministry of health; 2017.
Goldstein JA, Blaisdell J. [23] Genetic tests which identify the principal defects in CYP2C19 responsible for the polymorphism in mephenytoin metabolism. Methods Enzymol. 1996;272:210–8.
Nakamoto K, Kidd JR, Jenison RD, Klaassen CD, Wan Y-JY, Kidd KK, et al. Genotyping and haplotyping of CYP2C19 functional alleles on thin-film biosensor chips. Pharmacogenetics Genomics. 2007;17(2):103–14.
Ross KA, Bigham AW, Edwards M, Gozdzik A, Suarez-Kurtz G, Parra EJ. Worldwide allele frequency distribution of four polymorphisms associated with warfarin dose requirements. J Hum Genetics. 2010;55(9):582–9.
Ensembl-a. Ensembl release 89. EMBL-EBI, Wellcome Trust Genome Campus, Hinxton, Cambridge, United Kingdom. 2017. http://May2017.archive.ensembl.org/Homo_sapiens/Variation/Population?db=core;g=ENSG00000165841;r=10:94762624-94853260;v=rs4244285;vdb=variation;vf=2675574. Accessed 19 Oct 2018.
Buzoianu AD, Trifa AP, Popp RA, Militaru MS, Militaru CF, Bocşan CI, et al. Screening for CYP2C19* 2,* 3 and* 4 gene variants in a Romanian population study group. Farmacia. 2010;56:806–17.
Khalil BM, Shahin MH, Solayman MHM, Langaee T, Schaalan MF, Gong Y, et al. Genetic and nongenetic factors affecting clopidogrel response in the Egyptian population. Clin Transl Sci. 2016;9(1):23–8.
Saeed LH, Mayet AY. Genotype-phenotype analysis of CYP2C19 in healthy Saudi individuals and its potential clinical implication in drug therapy. Int J Med Sci. 2013;10(11):1497–502.
Jureidini ID, Chamseddine N, Keleshian S, Naoufal R, Zahed L, Hakime N. Prevalence of CYP2C19 polymorphisms in the Lebanese population. Mol Biol Rep. 2011;38(8):5449–52.
Zalloum I, Hakooz N, Arafat T. Genetic polymorphism of CYP2C19 in a Jordanian population: influence of allele frequencies of CYP2C19* 1 and CYP2C19* 2 on the pharmacokinetic profile of lansoprazole. Mol Biol Rep. 2012;39(4):4195–200.
Sviri S, Shpizen S, Leitersdorf E, Levy M, Caraco Y. Phenotypic-genotypic analysis of CYP2C19 in the Jewish Israeli population. Clin Pharmacol Ther. 1999;65(3):275–82.
Abid L, Laroussi L, Bahloul A, Siala A, Abdelhédi R, Kharrat N et al. Impact of cytochrome P450 2C19*2 polymorphism on the clinical cardiovascular events after stent implantation in patients receiving clopidogrel of a southern Tunisian region. World J Cardiovasc Dis. 2013;3(1):4–10.
Sameer A, Amany GM, Abdela AA, Fadel SA. CYP2C19 genotypes in a population of healthy volunteers and in children with hematological malignancies in Gaza Strip. Can J Clin Pharmacol. 2009;16(1):e156–62.
Nassar S, Amro O, Abu-Rmaileh H, Alshaer I, Korachi M, Ayesh S. ABCB1 C3435T and CYP2C19* 2 polymorphisms in a Palestinian and Turkish population: a pharmacogenetic perspective to clopidogrel. Meta gene. 2014;2:314–9.
Ensembl-b. Ensembl release 89. EMBL-EBI, Wellcome Trust Genome Campus, Hinxton, Cambridge, United Kingdom. 2017. http://May2017.archive.ensembl.org/Homo_sapiens/Variation/Population?db=core;r=10:94780153-94781153;v=rs4986893;vdb=variation;vf=3218155. Accessed 19 Oct 2018.
De Morais S, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol. 1994;46(4):594–8.
Al-Jenoobi FI, Alkharfy KM, Alghamdi AM, Bagulb KM, Al-Mohizea AM, Al-Muhsen S, et al. CYP2C19 genetic polymorphism in Saudi Arabians. Basic Clin Pharmacol Toxicol. 2013;112(1):50–4.
Zand N, Tajik N, Moghaddam AS, Milanian I. Genetic polymorphisms of cytochrome P450 enzymes 2C9 and 2C19 in a healthy Iranian population. Clin Exp Pharmacol Physiol. 2007;34(1–2):102–5.
Shahabi-Majd N, Habashi B. Frequencies of two CYP2C19 defective alleles (CYP2C19* 2, and* 3) among Iranian population in Mazandaran Province. Res Mol Med. 2013;1(1):17–21.
Goldstein JA, Ishizaki T, Chiba K, de Morais SM, Bell D, Krahn PM, et al. Frequencies of the defective CYP2C19 alleles responsible for the mephenytoin poor metabolizer phenotype in various Oriental, Caucasian, Saudi Arabian and American black populations. Pharmacogenetics Genomics. 1997;7(1):59–64.
Aynacioglu AS, Sachse C, Bozkurt A, Kortunay S, Nacak M, Schröder T, et al. Low frequency of defective alleles of cytochrome P450 enzymes 2C19 and 2D6 in the Turkish population. Clin Pharmacol Ther. 1999;66(2):185–92.
Sibbing D, Stegherr J, Latz W, Koch W, Mehilli J, Dörrler K et al. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur Heart J. 2009;30(8):916–22.
Wei YQ, Wang DG, Yang H, Cao H. Cytochrome P450 CYP 2C19*2 Associated with Adverse 1-Year Cardiovascular Events in Patients with Acute Coronary Syndrome. PLoS ONE. 2015;10(7):e0132561.
Guo B, Tan Q, Guo D, Shi Z, Zhang C, Guo W. Patients carrying CYP2C19 loss of function alleles have a reduced response to clopidogrel therapy and a greater risk of in-stent restenosis after endovascular treatment of lower extremity peripheral arterial disease. J Vasc Surg. 2014;60(4):993–1001.
Bauer T, Bouman HJ, van Werkum JW, Ford NF, ten Berg JM, Taubert D. Impact of CYP2C19 variant genotypes on clinical efficacy of antiplatelet treatment with clopidogrel: systematic review and meta-analysis. BMJ (Clinical research ed). 2011;343:d4588.
Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305(11):1097–105.
Zhang L, Yang J, Zhu X, Wang X, Peng L, Li X, et al. Effect of high-dose clopidogrel according to CYP2C19*2 genotype in patients undergoing percutaneous coronary intervention- a systematic review and meta-analysis. Thromb Res. 2015;135(3):449–58.
Chen Y, Zhang Y, Tang Y, Huang X, Xie Y. High-maintenance-dose clopidogrel in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. PLoS ONE. 2013;8(10):e78549.
Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. The Lancet. 2010;376(9748):1233–43.
Collet JP, Hulot JS, Anzaha G, Pena A, Chastre T, Caron C, et al. High doses of clopidogrel to overcome genetic resistance: the randomized crossover CLOVIS-2 (Clopidogrel and Response Variability Investigation Study 2). JACC Cardiovasc Interv. 2011;4(4):392–402.
Mathew V, Gersh BJ, Williams BA, Laskey WK, Willerson JT, Tilbury RT, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era. Circulation. 2004;109(4):476–80.
Bach R, Jung F, Kohsiek I, Özbek C, Spitzer S, Scheller B, et al. Factors affecting the restenosis rate after percutaneous transluminal coronary angioplasty. Thromb Res. 1994;74:S55–67.
Qin S-Y, Zhou Y, Jiang H-X, Hu B-L, Tao L, M-z Xie. The association of diabetes mellitus with clinical outcomes after coronary stenting: a meta-analysis. PLoS ONE. 2013;8(9):e72710.
Mohan S, Dhall A. A comparative study of restenosis rates in bare metal and drug-eluting stents. Int J Angiol. 2010;19(02):e66–72.
Kahn JK, Rutherford BD, McConahay DR, Johnson WL, Giorgi LV, Shimshak TM, et al. Comparison of procedural results and risks of coronary angioplasty in men and women for conditions other than acute myocardial infarction. Am J Cardiol. 1992;69(14):1241–2.
Bell MR, Holmes DR, Berger PB, Garratt KN, Bailey KR, Gersh BJ. The changing in-hospital mortality of women undergoing percutaneous transluminal coronary angioplasty. JAMA. 1993;269(16):2091–5.
Watanabe CT, Maynard C, Ritchie JL. Comparison of short-term outcomes following coronary artery stenting in men versus women. Am J Cardiol. 2001;88(8):848–52.
Jacobs AK, Johnston JM, Haviland A, Brooks MM, Kelsey SF, Holmes DR, et al. Improved outcomes for women undergoing contemporary percutaneous coronary intervention: a report from the National Heart, Lung, and Blood Institute Dynamic registry. J Am Coll Cardiol. 2002;39(10):1608–14.
Macdonald RG, Henderson MA, Hirshfeld JW, Goldberg SH, Bass T, Vetrovec G, et al. Patient-related variables and restenosis after percutaneous transluminal coronary angioplasty—a report from the M-HEART Groupt. Am J Cardiol. 1990;66(12):926–31.
Acknowledgements
The authors are grateful to the cardiac catheterization department of the European Gaza Hospital for facilitating the recruitment of patients.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ayesh, B.M., Al-Astal, I.R. & Yassin, M.M. The clinical effects of CYP2C19 *2 allele frequency on Palestinian patients receiving clopidogrel after percutaneous coronary intervention. Int J Clin Pharm 41, 96–103 (2019). https://doi.org/10.1007/s11096-018-00782-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-018-00782-3