Abstract
Purpose
In this study, we investigated in detail the transport of phenytoin across the blood-brain barrier (BBB) to identify the transporter(s) involved in BBB-mediated phenytoin efflux from the brain.
Methods
We evaluated the brain-to-blood efflux transport of phenytoin in vivo by determining the brain efflux index (BEI) and uptake in brain slices. We additionally conducted brain perfusion experiments and BEI studies in P-glycoprotein (P-gp)-deficient mice. In addition, we determined the mRNA expression of monocarboxylate transporter (MCT) in isolated brain capillaries and performed phenytoin uptake studies in MCT-expressing Xenopus oocytes.
Results
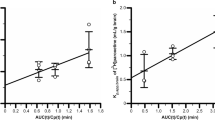
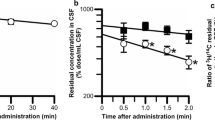
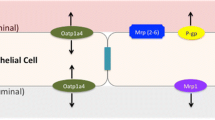
[14C]Phenytoin brain efflux was time-dependent with a half-life of 17 min in rats and 31 min in mice. Intracerebral pre-administration of unlabeled phenytoin attenuated BBB-mediated phenytoin efflux transport, suggesting carrier-mediated phenytoin efflux transport across the BBB. Pre-administration of P-gp substrates in rats and genetic P-gp deficiency in mice did not affect BBB-mediated phenytoin efflux transport. In contrast, pre-administration of MCT8 inhibitors attenuated phenytoin efflux. Moreover, rat MCT8-expressing Xenopus oocytes exhibited [14C]phenytoin uptake, which was inhibited by unlabeled phenytoin.
Conclusion
Our data suggest that MCT8 at the BBB participates in phenytoin efflux transport from the brain to the blood.








Similar content being viewed by others
Abbreviations
- ABC:
-
ATP-binding cassette
- BBB:
-
Blood-brain barrier
- BEI:
-
Brain efflux index
- BCRP:
-
Breast cancer resistance protein
- BSP:
-
Bromosulfophthalein
- CEF:
-
Cerebral extracellular fluid
- cDNA:
-
Complementary DNA
- cRNA:
-
Complementary RNA
- CYP:
-
Cytochrome P450
- DHEAS:
-
Dehydroepiandrosterone-3-sulfate
- HEPES:
-
4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid
- MCT:
-
Monocarboxylate transporter
- MRP:
-
Multidrug resistance-associated protein
- n :
-
Group sizes
- P-gp:
-
P-glycoprotein
- S.D.:
-
Standard deviation
- SNP:
-
Single nucleotide polymorphism
- X :
-
Xenopus
References
Zeng Z, Hill-Yardin EL, Williams D, O'Brien T, Serelis A, French CR. Effect of phenytoin on sodium conductances in rat hippocampal CA1 pyramidal neurons. J Neurophysiol. 2016;116(4):1924–36.
Carter CH. Use of parenteral diphenylhydantoin (dilantin) sodium in control of status epilepticus. AMA Arch Neurol Psychiatry. 1958;79(2):136–7.
Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–9.
Bajpai M, Roskos LK, Shen DD, Levy RH. Roles of cytochrome P4502C9 and cytochrome P4502C19 in the stereoselective metabolism of phenytoin to its major metabolite. Drug Metab Dispos. 1996;24(12):1401–3.
Fohner AE, Ranatunga DK, Thai KK, Lawson BL, Risch N, Oni-Orisan A, et al. Assessing the clinical impact of CYP2C9 pharmacogenetic variation on phenytoin prescribing practice and patient response in an integrated health system. Pharmacogenet Genomics. 2019;29(8):192–9.
Ramasamy K, Narayan SK, Shewade DG, Chandrasekaran A. Influence of CYP2C9 genetic polymorphism and undernourishment on plasma-free phenytoin concentrations in epileptic patients. Ther Drug Monit. 2010;32(6):762–6.
Cornford EM, Pardridge WM, Braun LD, Oldendorf WH. Increased blood--brain barrier transport of protein-bound anticonvulsant drugs in the newborn. J Cereb Blood Flow Metab. 1983;3(3):280–6.
Sadiq MW, Boström E, Keizer R, Bjorkman S, Hammarlund-Udenaes M. Oxymorphone active uptake at the blood–brain barrier and population modeling of its pharmacokinetic–Pharmacodynamic relationship. J Pharm Sci. 2013;102(9):3320–31.
Ohtsuki S, Terasaki T. Contribution of carrier-mediated transport systems to the blood-brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res. 2007;24(9):1745–58.
Okura T, Hattori A, Takano Y, Sato T, Hammarlund-Udenaes M, Terasaki T, et al. Involvement of the pyrilamine transporter, a putative organic cation transporter, in blood-brain barrier transport of oxycodone. Drug Metab Dispos. 2008;36(10):2005–13.
Potschka H, Loscher W. In vivo evidence for P-glycoprotein-mediated transport of phenytoin at the blood-brain barrier of rats. Epilepsia. 2001;42(10):1231–40.
Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97(11):2517–24.
Felmlee MA, Jones RS, Rodriguez-Cruz V, Follman KE, Morris ME. Monocarboxylate transporters (SLC16): function, regulation, and role in health and disease. Pharmacol Rev. 2020;72(2):466–85.
Roberts LM, Woodford K, Zhou M, Black DS, Haggerty JE, Tate EH, et al. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology. 2008;149(12):6251–61.
Roth S, Kinne A, Schweizer U. The tricyclic antidepressant desipramine inhibits T3 import into primary neurons. Neurosci Lett. 2010;478(1):5–8.
Kakee A, Terasaki T, Sugiyama Y. Brain efflux index as a novel method of analyzing efflux transport at the blood-brain barrier. J Pharmacol Exp Ther. 1996;277(3):1550–9.
Akanuma S, Ohtsuki S, Doi Y, Tachikawa M, Ito S, Hori S, et al. ATP-binding cassette transporter A1 (ABCA1) deficiency does not attenuate the brain-to-blood efflux transport of human amyloid-beta peptide (1-40) at the blood-brain barrier. Neurochem Int. 2008;52(6):956–61.
Akanuma S, Higuchi T, Higashi H, Ozeki G, Tachikawa M, Kubo Y, et al. Transporter-mediated prostaglandin E(2) elimination across the rat blood-brain barrier and its attenuation by the activation of N-methyl-D-aspartate receptors. Drug Metab Pharmacokinet. 2014;29(5):387–93.
Urayama A, Grubb JH, Sly WS, Banks WA. Pharmacologic manipulation of lysosomal enzyme transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2016;36(3):476–86.
Soldner ELB, Hartz AMS, Akanuma SI, Pekcec A, Doods H, Kryscio RJ, et al. Inhibition of human microsomal PGE2 synthase-1 reduces seizure-induced increases of P-glycoprotein expression and activity at the blood-brain barrier. FASEB J. 2019;33(12):13966–81.
Akanuma SI, Yamakoshi A, Sugouchi T, Kubo Y, Hartz AMS, Bauer B, et al. Role of L-type amino acid transporter 1 at the inner blood-retinal barrier in the blood-to-retina transport of gabapentin. Mol Pharm. 2018;15(6):2327–37.
Akanuma S, Kida R, Tsuchiyama A, Tachikawa M, Kubo Y, Hosoya K. Organic anion-transporting polypeptide 1a4-mediated heterogeneous distribution of sulforhodamine-101 in rat hepatic lobules. Drug Metab Pharmacokinet. 2019;34(4):239–46.
Cui Y, Konig J, Keppler D. Vectorial transport by double-transfected cells expressing the human uptake transporter SLC21A8 and the apical export pump ABCC2. Mol Pharmacol. 2001;60(5):934–43.
Kusuhara H, Suzuki H, Terasaki T, Kakee A, Lemaire M, Sugiyama Y. P-glycoprotein mediates the efflux of quinidine across the blood-brain barrier. J Pharmacol Exp Ther. 1997;283(2):574–80.
Grube M, Hagen P, Jedlitschky G. Neurosteroid transport in the brain: role of ABC and SLC transporters. Front Pharmacol. 2018;9:354.
Matsson P, Pedersen JM, Norinder U, Bergstrom CA, Artursson P. Identification of novel specific and general inhibitors of the three major human ATP-binding cassette transporters P-gp, BCRP and MRP2 among registered drugs. Pharm Res. 2009;26(8):1816–31.
Murakami Y, Kohyama N, Kobayashi Y, Ohbayashi M, Ohtani H, Sawada Y, et al. Functional characterization of human monocarboxylate transporter 6 (SLC16A5). Drug Metab Dispos. 2005;33(12):1845–51.
Zamek-Gliszczynski MJ, Xiong H, Patel NJ, Turncliff RZ, Pollack GM, Brouwer KL. Pharmacokinetics of 5 (and 6)-carboxy-2′,7′-dichlorofluorescein and its diacetate promoiety in the liver. J Pharmacol Exp Ther. 2003;304(2):801–9.
Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem. 2003;278(41):40128–35.
Schutkowski A, Wege N, Stangl GI, Konig B. Tissue-specific expression of monocarboxylate transporters during fasting in mice. PLoS One. 2014;9(11):e112118.
Bankstahl M, Klein S, Romermann K, Loscher W. Knockout of P-glycoprotein does not alter antiepileptic drug efficacy in the intrahippocampal kainate model of mesial temporal lobe epilepsy in mice. Neuropharmacology. 2016;109:183–95.
El-Sheikh AA, Koenderink JB, Wouterse AC, van den Broek PH, Verweij VG, Masereeuw R, et al. Renal glucuronidation and multidrug resistance protein 2−/ multidrug resistance protein 4-mediated efflux of mycophenolic acid: interaction with cyclosporine and tacrolimus. Transl Res. 2014;164(1):46–56.
Vijay N, Morris ME. Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharm Des. 2014;20(10):1487–98.
Fisel P, Schaeffeler E, Schwab M. Clinical and functional relevance of the monocarboxylate transporter family in disease pathophysiology and drug therapy. Clin Transl Sci. 2018;11(4):352–64.
Muzzio AM, Noyes PD, Stapleton HM, Lema SC. Tissue distribution and thyroid hormone effects on mRNA abundance for membrane transporters Mct8, Mct10, and organic anion-transporting polypeptides (Oatps) in a teleost fish. Comp Biochem Physiol A Mol Integr Physiol. 2014;167:77–89.
Wilpert NM, Krueger M, Opitz R, Sebinger D, Paisdzior S, Mages B, et al. Spatiotemporal changes of cerebral monocarboxylate transporter 8 expression. Thyroid. 2020;30(9):1366–83.
Muller J, Heuer H. Expression pattern of thyroid hormone transporters in the postnatal mouse brain. Front Endocrinol (Lausanne). 2014;5:92.
Roef GL, Rietzschel ER. De Meyer T, Bekaert S, De Buyzere ML, Van daele C, Toye K, Kaufman JM, Taes YE. Associations between single nucleotide polymorphisms in thyroid hormone transporter genes (MCT8, MCT10 and OATP1C1) and circulating thyroid hormones. Clin Chim Acta. 2013;425:227–32.
Mayerl S, Muller J, Bauer R, Richert S, Kassmann CM, Darras VM, et al. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J Clin Invest. 2014;124(5):1987–99.
Miyake Z, Ishii K, Tamaoka A. Hypothyroidism induced by phenytoin and gabapentin: a case report. Medicine (Baltimore). 2018;97(43):e12938.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors thank Mr. Kosuke Tajima (University of Toyama) for helping with the mRNA expression analysis for MCTs. This research was supported by The Mochida Memorial Foundation for Medical and Pharmaceutical Research, The Research Foundation for Pharmaceutical Sciences, and Japan Society for the Promotion of Science (JSPS) KAKENHI [Grant Numbers JP16K08365 (to S.A.), JP16H05110 (to K.H.), and JP19K07160 (to S.A.)]. This project was also supported by Grant 1R01NS079507 from the U.S. National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (to B.B.). The content is solely the responsibility of the authors, and it does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the NIH. All authors declare no conflict of interest.
Author information
Authors and Affiliations
Contributions
R.J., S.A., B.B., and Y.Y. performed the experiments; R.J., S.A., B.B., Y.K., and H.K. designed the experiments and analyzed the data.; and R.J. and S.A. wrote the manuscript. All authors read the final version of the manuscript.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jomura, R., Akanuma, Si., Bauer, B. et al. Participation of Monocarboxylate Transporter 8, But Not P-Glycoprotein, in Carrier-Mediated Cerebral Elimination of Phenytoin across the Blood-Brain Barrier. Pharm Res 38, 113–125 (2021). https://doi.org/10.1007/s11095-021-03003-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-03003-1