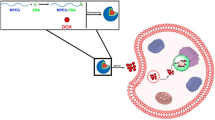
Abstract
Purpose
The selective delivery of chemotherapeutic agent to the affected area is mainly dependent on the mode of drug loading within the delivery system. This study aims to compare the physical method to the chemical method on the efficiency of loading DOX.HCl to GNPs and the specific release of the loaded drug at certain tissue.
Method
Bifunctional polyethylene glycol with two different functionalities, the alkanethiol and the carboxyl group terminals, was synthesized. Then, DOX·HCl was covalently linked via hydrazone bond, a pH sensitive bond, to the carboxyl functional group and the produced polymer was used to prepare drug functionalized nanoparticles. Another group of GNPs was coated with carboxyl containing polymer; loading the drug into this system by the means of electrostatic adsorption. Finally, the prepared system were characterized with respect to size, shape and drug release in acetate buffer pH 5 and PBS pH 7.4 Also, the effect of DOX.HCl loaded systems on cell viability was assessed using MCF-7 breast cancer cell line.
Results
The prepared nanoparticles were spherical in shape, small in size and monodisperse. The release rate of the chemically bound drug in the acidic pH was higher than the electrostatically adsorbed one. Moreover, both systems show little release at pH 7.4. Finally, cytotoxicity profiles against human breast adenocarcinoma cell line (MCF-7) exhibited greater cytotoxicity of the chemically bound drug over the electrostatically adsorbed one.
Conclusion
Chemical binding of DOX·HCl to the carboxyl group of PEG coating GNPs selectively delivers high amount of drug to tumour-affected tissue which leads to reducing the unwanted effects of the drug in the non-affected ones.







Similar content being viewed by others
Abbreviations
- AIBN:
-
Azobisisobutronitrile
- ANOVA:
-
Analysis of variance
- DCM:
-
Dichloromethane
- DOX·HCl :
-
Doxorubicin HCl
- DMSO:
-
Dimethyl sulfoxide
- EPR:
-
Enhanced permeability and retention
- GNPs:
-
Gold nanoparticles
- HAuCl4.3 H3O:
-
Hydrogen tetrachloroauric acid trihydrate
- IC50 :
-
The concentration of substance required for 50% growth inhibition
- MCF-7:
-
Breast cancer cell line
- MDR:
-
Multi-drug resistance
- MWCO:
-
Molecular weight cut off
- PCS:
-
Photon correlation spectroscopy
- PDI:
-
Polydispersity index
- P-gp:
-
P-glycoprotein
- PBS:
-
Phosphate buffered saline
- PEG:
-
Polyethylene glycol
- RES:
-
Reticulo-endothelial system
- SPR:
-
Surface plasmon resonance
- TEM:
-
Transmission electron microscope
References
Clarke R, Leonessa F, Multidrug TB. Resistance/P-glycoprotein and breast Cancer: Review and meta-analysis. Semin Oncol. 2005;32(Supplement 7):9–15.
Wang J, Wu L, Kou L, Xu M, Sun J, Wang Y, et al. Novel nanostructured enoxaparin sodium-PLGA hybrid carriers overcome tumor multidrug resistance of doxorubicin hydrochloride. Int J Pharm. 2016;513(1):218–26.
Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54(5):631–51.
Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1(3):325–7.
Ruan S, Yuan M, Zhang L, Hu G, Chen J, Cun X, et al. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials. 2015;37:425–35.
Choi SH, Byeon HJ, Choi JS, Thao L, Kim I, Lee ES, et al. Inhalable self-assembled albumin nanoparticles for treating drug-resistant lung cancer. J Control Release. 2015;197:199–207.
Zhang G, Yang Z, Lu W, Zhang R, Huang Q, Tian M, et al. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials. 2009;30(10):1928–36.
Gao J, Huang X, Liu H, Zan F, Ren J. Colloidal stability of gold nanoparticles modified with thiol compounds: Bioconjugation and application in Cancer cell imaging. Langmuir. 2012;28(9):4464–71.
Liu X, Huang N, Wang H, Li H, Jin Q, Ji J. The effect of ligand composition on the in vivo fate of multidentate poly(ethylene glycol) modified gold nanoparticles. Biomaterials. 2013;34(33):8370–81.
Bednarski M, Dudek M, Knutelska J, Nowiński L, Sapa J, Zygmunt M, et al. The influence of the route of administration of gold nanoparticles on their tissue distribution and basic biochemical parameters: in vivo studies. Pharmacol Rep. 2015;67(3):405–9.
Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, et al. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16(25):3267–85.
Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, et al. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21(7):440–6.
Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H. Nanoparticles as drug delivery systems. Pharmacol Rep. 2012;64(5):1020–37.
del Rosario LS, Demirdirek B, Harmon A, Orban D, Uhrich KE. Micellar Nanocarriers assembled from doxorubicin-conjugated amphiphilic macromolecules (DOX–AM). Macromol Biosci. 2010;10(4):415–23.
Guerzoni LPB, Nicolas V, Angelova A. In vitro modulation of TrkB receptor signaling upon sequential delivery of curcumin-DHA loaded carriers towards promoting neuronal survival. Pharm Res. 2017;34(2):492–505.
Angelova A, Garamus VM, Angelov B, Tian Z, Li Y, Zou A. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv Colloid Interf Sci. 2017;249:331–45.
Angelova A, Angelov B, Drechsler M, Lesieur S. Neurotrophin delivery using nanotechnology. Drug Discov Today. 2013;18(23):1263–71.
Chen Y, Angelova A, Angelov B, Drechsler M, Garamus VM, Willumeit-Romer R, et al. Sterically stabilized spongosomes for multidrug delivery of anticancer nanomedicines. J Mater Chem B. 2015;3(39):7734–44.
Zerkoune L, Lesieur S, Putaux J-L, Choisnard L, Geze A, Wouessidjewe D, et al. Mesoporous self-assembled nanoparticles of biotransesterified cyclodextrins and nonlamellar lipids as carriers of water-insoluble substances. Soft Matter. 2016;12(36):7539–50.
Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, et al. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Delivery. 2004;11(3):169–83.
El-Sayed IH, Huang X, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006;239(1):129–35.
Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11(0):55–75.
Che E, Wan L, Zhang Y, Zhao Q, Han X, Li J, et al. Development of phosphonate-terminated magnetic mesoporous silica nanoparticles for pH-controlled release of doxorubicin and improved tumor accumulation. Asian J Pharm Sci. 2014;9(6):317–23.
Mahou R, Wandrey C. Versatile route to synthesize heterobifunctional poly (ethylene glycol) of variable functionality for subsequent pegylation. Polymers. 2012;4(1):561–89.
Shenoy D, Fu W, Li J, Crasto C, Jones G, DiMarzio C, et al. Surface functionalization of gold nanoparticles using hetero-bifunctional poly (ethylene glycol) spacer for intracellular tracking and delivery. Int J Nanomedicine. 2006;1(1):51–8.
Woghiren C, Sharma B, Stein S. Protected thiol-polyethylene glycol: a new activated polymer for reversible protein modification. Bioconjug Chem. 1993;4(5):314–8.
Napoli A, Tirelli N, Kilcher G, Hubbell A. New synthetic methodologies for amphiphilic multiblock copolymers of ethylene glycol and propylene sulfide. Macromolecules. 2001;34(26):8913–7.
Volcker N, Klee D, Hanna M, Hocker H, Bou JJ, Ilarduya Ad, et al. Synthesis of heterotelechelic poly (ethylene glycol) s and their characterization by MALDI-TOF-MS. Macromol Chem Phys 1999;200(6):1363–1373.
Bogdanov AA. Graft-copolymer stabilized metal nanoparticles. US patent. 2015;
Moldt T, Brete D, Przyrembel D, Das S, Goldman JR, Kundu PK, et al. Tailoring the properties of surface-immobilized Azobenzenes by monolayer dilution and surface curvature. Langmuir. 2015;31(3):1048–57.
Overberger C, O'shaughnessy M, Shalit H. The preparation of some aliphatic azo nitriles and their decomposition in solution. J Am Chem Soc. 1949;71(8):2661–6.
Herrwerth S, Rosendahl T, Feng C, Fick J, Eck W, Himmelhaus M, et al. Covalent coupling of antibodies to self-assembled monolayers of Carboxy-functionalized poly(ethylene glycol): protein resistance and specific binding of biomolecules. Langmuir. 2003;19(5):1880–7.
Zayed G, Tessmar JK. Heterobifunctional poly (ethylene glycol) derivatives for the surface modification of gold nanoparticles toward bone mineral targeting. Macromol Biosci. 2012;12(8):1124–36.
Prime KL, Whitesides GM. Adsorption of proteins onto surfaces containing end-attached oligo(ethylene oxide): a model system using self-assembled monolayers. J Am Chem Soc. 1993;115(23):10714–21.
Li JJ. Williamson ether synthesis. Name Reactions: Springer; 2014. p. 628-.
Witt D, Klajn R, Barski P, Grzybowski BA. Applications, properties and synthesis of Ѡ-functionalized n-Alkanethiols and disulfides - the building blocks of self-assembled monolayers. Curr Org Chem. 2004;8(18):1763–97.
Nudelman A, Bechor Y, Falb E, Fischer B, Wexler BA, Nudelman A. Acetyl chloride-methanol as a convenient reagent for: A) quantitative formation of amine hydrochlorides B) carboxylate Ester formation C) mild removal of N-t-Boc-protective group. Synth Commun. 1998;28(3):471–474.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–7.
Tyuftin AA, Solovieva SE, Murav’ev AA, Polyantsev FM, Latypov SK, Antipina IS. Thiacalix[4] arenes with terminal thiol groups at the lower rim: synthesis and structure. Russ Chem Bull. 2009;58(1):145–51.
Patil R, Portilla-Arias J, Ding H, Konda B, Rekechenetskiy A, Inoue S, et al. Cellular delivery of doxorubicin via pH-controlled hydrazone linkage using multifunctional nano vehicle based on poly (β-L-malic acid). Int J Mol Sci. 2012;13(9):11681–93.
Wang F, Wang Y-C, Dou S, Xiong M-H, Sun T-M, Wang J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in Cancer cells. ACS Nano. 2011;5(5):3679–92.
Etrych T, Mrkvan T, Chytil P, Koňák Č, Říhová B, Ulbrich K. N-(2-hydroxypropyl)methacrylamide-based polymer conjugates with pH-controlled activation of doxorubicin. I. New synthesis, physicochemical characterization and preliminary biological evaluation. J Appl Polym Sci. 2008;109(5):3050–61.
Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature. 1973;241(105):20–2.
Madhusudhan A, Reddy GB, Venkatesham M, Veerabhadram G, Kumar DA, Natarajan S, et al. Efficient pH dependent drug delivery to target cancer cells by gold nanoparticles capped with carboxymethyl chitosan. Int J Mol Sci. 2014;15(5):8216–34.
Gu Y-J, Cheng J, Man CW-Y, Wong W-T, Cheng SH. Gold-doxorubicin nanoconjugates for overcoming multidrug resistance. Nanomedicine. 2012;8(2):204–11.
Corbierre MK, Cameron NS, Lennox RB. Polymer-stabilized gold nanoparticles with high grafting densities. Langmuir. 2004;20(7):2867–73.
Vigevani A, Doxorubicin WMJ. Doxorubicin. In: Klaus F, editor. Analytical profiles of drug substances. Volume 9: academic press; 1981. p. 245–74.
Norman TJ, Grant CD, Magana D, Zhang JZ, Liu J, Cao D, et al. Near infrared optical absorption of gold nanoparticle aggregates. J Phys Chem B. 2002;106(28):7005–12.
Chah S, Hammond MR, Zare RN. Gold nanoparticles as a colorimetric sensor for protein conformational changes. Chem Biol. 12(3):323–8.
Burns C, Spendel WU, Puckett S, Pacey GE. Solution ionic strength effect on gold nanoparticle solution color transition. Talanta. 2006;69(4):873–6.
Nam J, Won N, Jin H, Chung H, Kim S. pH-induced aggregation of gold nanoparticles for Photothermal Cancer therapy. J Am Chem Soc. 2009;131(38):13639–45.
Aryal S, RB KC, Bhattarai N, Kim CK, Kim HY. Study of electrolyte induced aggregation of gold nanoparticles capped by amino acids. J Colloid Interface Sci. 2006;299(1):191–7.
Wang G, Sun W. Optical limiting of gold nanoparticle aggregates induced by electrolytes. J Phys Chem B. 2006;110(42):20901–5.
Takae S, Akiyama Y, Otsuka H, Nakamura T, Nagasaki Y, Kataoka K. Ligand density effect on Biorecognition by PEGylated gold nanoparticles: regulated interaction of RCA120 lectin with lactose installed to the distal end of tethered PEG strands on gold surface. Biomacromolecules. 2005;6(2):818–24.
Jo DH, Kim JH, Lee TG, Kim JH. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine. 2015;11(7):1603–11.
Chavanpatil MD, Khdair A, Patil Y, Handa H, Mao G, Panyam J. Polymer-surfactant nanoparticles for sustained release of water-soluble drugs. J Pharm Sci. 2007;96(12):3379–89.
Zayed G, Tessmar J, Gopferich A. Development of colloids for cell and tissue targeting: bisphosphonate-functionalized gold nanoparticles for the investigation of bone targeting. In: phD; 2010.
Miyamoto D, Oishi M, Kojima K, Yoshimoto K, Nagasaki Y. Completely dispersible PEGylated gold nanoparticles under physiological conditions: modification of gold nanoparticles with precisely controlled PEG-b-polyamine. Langmuir. 2008;24(9):5010–7.
Lu D, Wen X, Liang J, Gu Z, Zhang X, Fan Y. A pH-sensitive nano drug delivery system derived from pullulan/doxorubicin conjugate. J Biomed Mater Res B Appl Biomater. 2009;89B(1):177–83.
Sun T-M, Wang Y-C, Wang F, Du J-Z, Mao C-Q, Sun C-Y, et al. Cancer stem cell therapy using doxorubicin conjugated to gold nanoparticles via hydrazone bonds. Biomaterials. 2014;35(2):836–45.
Zhang Z, Wang L, Wang J, Jiang X, Li X, Hu Z, et al. Mesoporous silica-coated gold Nanorods as a light-mediated multifunctional Theranostic platform for Cancer treatment. Adv Mater. 2012;24(11):1418–23.
François A, Laroche A, Pinaud N, Salmon L, Ruiz J, Robert J, et al. Encapsulation of docetaxel into PEGylated gold nanoparticles for vectorization to Cancer cells. ChemMedChem. 2011;6(11):2003–8.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 1034 kb)
Rights and permissions
About this article
Cite this article
Zayed, G.M., Kamal, I., Abdelhafez, W.A. et al. Effect of Chemical Binding of Doxorubicin Hydrochloride to Gold Nanoparticles, Versus Electrostatic Adsorption, on the In Vitro Drug Release and Cytotoxicity to Breast Cancer Cells. Pharm Res 35, 112 (2018). https://doi.org/10.1007/s11095-018-2393-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2393-6