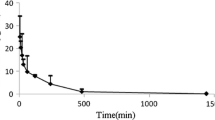
Abstract
Purpose
The objective was the development of a whole-body physiologically-based pharmacokinetic (WB-PBPK) model for colistin, and its prodrug colistimethate sodium (CMS), in pigs to explore their tissue distribution, especially in kidneys.
Methods
Plasma and tissue concentrations of CMS and colistin were measured after systemic administrations of different dosing regimens of CMS in pigs. The WB-PBPK model was developed based on these data according to a non-linear mixed effect approach and using NONMEM software. A detailed sub-model was implemented for kidneys to handle the complex disposition of CMS and colistin within this organ.
Results
The WB-PBPK model well captured the kinetic profiles of CMS and colistin in plasma. In kidneys, an accumulation and slow elimination of colistin were observed and well described by the model. Kidneys seemed to have a major role in the elimination processes, through tubular secretion of CMS and intracellular degradation of colistin. Lastly, to illustrate the usefulness of the PBPK model, an estimation of the withdrawal periods after veterinary use of CMS in pigs was made.
Conclusions
The WB-PBPK model gives an insight into the renal distribution and elimination of CMS and colistin in pigs; it may be further developed to explore the colistin induced-nephrotoxicity in humans.










Similar content being viewed by others
Abbreviations
- ADME:
-
Absorption, distribution, metabolism, excretion
- BLOQ:
-
Below the limit of quantification
- BW:
-
Body weight
- CBA:
-
Colistin base activity
- CMS:
-
Colistimethate sodium
- DV:
-
Observed value
- fu:
-
Unbound fraction
- GFR:
-
Glomerular filtration rate
- GIT:
-
Gastro-intestinal tract
- HPLC-MS/MS:
-
High-performance liquid chromatography coupled with tandem mass spectrometry
- IIV:
-
Interindividual variability
- IM:
-
Intramuscular
- IPRED:
-
Individual prediction
- IV:
-
Intravenous
- LOQ:
-
Limit of quantification
- MRL:
-
Maximal residue limits
- NLME:
-
Nonlinear mixed effects
- OFV:
-
Objective function value
- PBPK:
-
Physiologically-based pharmacokinetic
- PK:
-
Pharmacokinetics
- PRED:
-
Population prediction
- RV:
-
Residual variability
- SIR:
-
Sampling importance resampling
- t1/2 :
-
Half-life
- VPC:
-
Visual predictive checks
- WB-PBPK:
-
Whole body physiologically-based pharmacokinetic
- WP:
-
Withdrawal period
References
Gregoire N, Aranzana-Climent V, Magreault S, Marchand S, Couet W. Clinical pharmacokinetics and pharmacodynamics of Colistin. Clin Pharmacokinet. 2017;56:1441–60.
Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother. 2004;53(5):837–40.
Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K. Stability of Colistin and Colistin Methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob Agents Chemother. 2003;47(4):1364–70.
Forrest A, Garonzik SM, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, Li J, et al. Pharmacokinetic/toxicodynamic analysis of colistin-associated acute kidney injury in critically ill patients. Antimicrobial Agents and Chemotherapy. 2017:AAC. 01367–17.
Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. Evaluation of colistin as an agent against multi-resistant gram-negative bacteria. Int J Antimicrob Agents. 2005;25(1):11–25.
Couet W, Gregoire N, Gobin P, Saulnier P, Frasca D, Marchand S, et al. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin Pharmacol Ther. 2011;89(6):875–9.
Craig WA, Kunin CM. Dynamics of binding and release of the polymyxin antibiotics by tissues. J Pharmacol Exp Ther. 1973;184(3):757–65.
Tomasi L, Giovannetti L, Rondolotti A, Rocca GD, Stracciari GL. Depletion of the residues of colistin and amoxicillin in turkeys following simultaneous subcutaneous administration. Vet Res Commun. 1996;20(2):175–82.
Ma Z, Wang J, Nation RL, Li J, Turnidge JD, Coulthard K, et al. Renal disposition of colistin in the isolated perfused rat kidney. Antimicrob Agents Chemother. 2009;53(7):2857–64.
Suzuki T, Yamaguchi H, Ogura J, Kobayashi M, Yamada T, Iseki K. Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob Agents Chemother. 2013;57(12):6319–24.
Yun B, Azad MA, Wang J, Nation RL, Thompson PE, Roberts KD, et al. Imaging the distribution of polymyxins in the kidney. J Antimicrob Chemother. 2015:70(3):827–9.
Yun B, Azad MA, Nowell CJ, Nation RL, Thompson PE, Roberts KD, et al. Cellular uptake and localization of polymyxins in renal tubular cells using rationally designed fluorescent probes. Antimicrob Agents Chemother. 2015;59(12):7489–96.
Azad MA, Roberts KD, Yu HH, Liu B, Schofield AV, James SA, et al. Significant accumulation of polymyxin in single renal tubular cells: a medicinal chemistry and triple correlative microscopy approach. Anal Chem. 2015;87(3):1590–5.
Lu X, Chan T, Xu C, Zhu L, Zhou QT, Roberts KD, et al. Human oligopeptide transporter 2 (PEPT2) mediates cellular uptake of polymyxins. J Antimicrob Chemother. 2016;71(2):403–12.
Dai C, Li J, Tang S, Li J, Xiao X. Colistin-induced nephrotoxicity in mice involves the mitochondrial, death receptor, and endoplasmic reticulum pathways. Antimicrob Agents Chemother. 2014;58(7):4075–85.
Azad MA, Akter J, Rogers K, Nation RL, Velkov T, Li J. Major pathways of polymyxin-induced apoptosis in rat kidney proximal tubular cells. Antimicrob Agents Chemother. 2015;59(4):2136–43.
Nestorov I. Whole-body physiologically based pharmacokinetic models. Expert Opin Drug Metab Toxicol. 2007;3(2):235–49.
Bouchene S, Marchand S, Couet W, Friberg LE, Gobin P, Lamarche I, et al. Comparison of Colistin and Colistimethate sodium (CMS) model-predicted whole-body distribution with measured tissue:plasma concentrations ratios in rats. 53rd interscience conference on antimicrobial agents and chemotherapy; Denver, Co 2013.
Sheiner LB, Beal SL. Evaluation of methods for estimating population pharmacokinetic parameters II. Biexponential model and experimental pharmacokinetic data. J Pharmacokinet Pharmacodyn. 1981;9(5):635–51.
Swindle MM, Makin A, Herron AJ, Clubb FJ, Frazier KS. Swine as models in biomedical research and toxicology testing. Veterinary Pathology Online. 2012;49(2):344–56.
JECFA. Residue evaluation of certain veterinary drugs: joint FAO/WHO expert committee on food additives, 66th meeting 2006: Food & Agriculture org.; 2006.
EMA. Guideline on approach towards harmonisation of withdrawal periods. European Medicines Agency (EMA) - Committee for Medicinal Products for Veterinary Use (CVMP), 2016 EMA/CVMP/CHMP/231573/2016 Contract No.: EMA/CVMP/SWP/735325/2012.
Lin Z, Gehring R, Mochel J, Lavé T, Riviere J. Mathematical modeling and simulation in animal health–part II: principles, methods, applications, and value of physiologically based pharmacokinetic modeling in veterinary medicine and food safety assessment. J Vet Pharmacol Ther. 2016;39(5):421–38.
Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, et al. Consistent global approach on reporting of Colistin doses to promote safe and effective use. Clin Infect Dis. 2014;58(1):139–41.
Rottbøll LAH, Friis C. Penetration of antimicrobials to pulmonary epithelial lining fluid and muscle and impact of drug physicochemical properties determined by microdialysis. J Pharmacol Toxicol Methods. 2016;78:58–65.
Gobin P, Lemaître F, Marchand S, Couet W, Olivier J-C. Assay of Colistin and Colistin Methanesulfonate in plasma and urine by liquid chromatography-tandem mass spectrometry. Antimicrob Agents Chemother. 2010;54(5):1941–8.
Buur JL, Baynes RE, Craigmill AL, Riviere JE. Development of a physiologic-based pharmacokinetic model for estimating sulfamethazine concentrations in swine and application to prediction of violative residues in edible tissues. Am J Vet Res. 2005;66(10):1686–93.
Chen K, Calibration SKY. Validation of a physiologically based model for soman intoxication in the rat, marmoset, Guinea pig and pig. J Appl Toxicol. 2012;32(9):673–86.
de Boer VC, Dihal AA, van der Woude H, Arts IC, Wolffram S, Alink GM, et al. Tissue distribution of quercetin in rats and pigs. J Nutr. 2005;135(7):1718–25.
Dubbelboer IR, Lilienberg E, Sjögren E, Lennernas H. A model-based approach to assessing the importance of intracellular binding sites in doxorubicin disposition. Mol Pharm. 2017;14(3):686–98.
Elowsson P, Carlsten J. Body composition of the 12-week-old pig studied by dissection. Lab Anim Sci. 1997;47(2):200–2.
Eskild-Jensen A, Jacobsen L, Christensen H, Frokiaer J, Jorgensen HS, Djurhuus JC, et al. Renal function outcome in unilateral hydronephrosis in newborn pigs. II. Function and volume of contralateral kidneys. J Urol. 2001;165(1):205–9.
Lødrup AB, Karstoft K, Dissing TH, Nyengaard JR, Pedersen M. The association between renal function and structural parameters: a pig study. BMC Nephrol. 2008;9:18.
Lundeen G, Manohar M, Parks C. Systemic distribution of blood flow in swine while awake and during 1.0 and 1.5 MAC isoflurane anesthesia with or without 50% nitrous oxide. Anesth Analg. 1983;62(5):499–512.
Rendas A, Branthwaite M, Reid L. Growth of pulmonary circulation in normal pig--structural analysis and cardiopulmonary function. J Appl Physiol. 1978;45(5):806–17.
Scotcher D, Jones C, Posada M, Rostami-Hodjegan A, Galetin A. Key to opening kidney for in vitro–in vivo extrapolation entrance in health and disease: part I: in vitro systems and physiological data. AAPS J. 2016;18(5):1067–81.
Suenderhauf C, Parrott N. A physiologically based pharmacokinetic model of the minipig: data compilation and model implementation. Pharm Res. 2013;30(1):1–15.
Ten have GAM, Bost MCF, Suyk-Wierts JCAW, van den Bogaard AEJM, Deutz NEP. Simultaneous measurement of metabolic flux in portally-drained viscera, liver, spleen, kidney and hindquarter in the conscious pig. Lab Anim. 1996;30(4):347–58.
Tranquilli WJ, Manohar M, Parks CM, Thurmon JC, Theodorakis MC, Systemic BGJ. Regional blood flow distribution in unanesthetized swine and swine anesthetized with halothane + nitrous oxide, halothane, or enflurane. Anesthesiology. 1982;56(5):369–79.
Upton RN. Organ weights and blood flows of sheep and pig for physiological pharmacokinetic modelling. J Pharmacol Toxicol Methods. 2008;58(3):198–205.
Vinegar A. Development of a physiologically based pharmacokinetic model for the anesthetics halothane, isoflurane, and desflurane in the pig (Sus scrofa). DTIC Document, 1999.
Drougas JG, Barnard SE, Wright JK, Sika M, Lopez RR, Stokes KA, et al. A model for the extended studies of hepatic hemodynamics and metabolism in swine. Lab Anim Sci. 1996;46(6):648–55.
Peters SA. Physiologically-based pharmacokinetic (PBPK) modeling and simulations: principles, methods, and applications in the pharmaceutical industry. New York: Wiley; 2012.
Scotcher D, Jones C, Rostami-Hodjegan A, Galetin A. Novel minimal physiologically-based model for the prediction of passive tubular reabsorption and renal excretion clearance. Eur J Pharm Sci. 2016;94:59–71.
Leavens T, Tell L, Clothier K, Griffith R, Baynes RE, Riviere J. Development of a physiologically based pharmacokinetic model to predict tulathromycin distribution in goats. J Vet Pharmacol Ther. 2012;35(2):121–31.
Dosne A-G, Bergstrand M, Harling K, Karlsson MO. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J Pharmacokinet Pharmacodyn. 2016;43(6):583–96.
Karlsson MO, Beal SL, Sheiner LB. Three new residual error models for population PK/PD analyses. J Pharmacokinet Pharmacodyn. 1995;23(6):651–72.
Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481–504.
Chevance A, Jacques AM, Laurentie M, Sanders P, Henri J. The present and future of withdrawal period calculations for milk in the European Union: focus on heterogeneous, nonmonotonic data. J Vet Pharmacol Ther. 2017;40(3):218–30.
Lindbom L, Pihlgren P, Jonsson N. PsN-toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Prog Biomed. 2005;79(3):241–57.
Keizer RJ, Van Benten M, Beijnen JH, Schellens JH, Huitema AD. Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Prog Biomed. 2011;101(1):72–9.
Landersdorfer CB, Nguyen T-H, Lieu LT, Nguyen G, Bischof RJ, Meeusen EN, et al. Substantial targeting advantage achieved by pulmonary Administration of Colistin Methanesulfonate in a large-animal model. Antimicrob Agents Chemother. 2017;61(1):e01934–16.
Karvanen M, Malmberg C, Lagerback P, Friberg LE, Cars O. Colistin is extensively lost during standard in vitro experimental conditions. Antimicrob Agents Chemother. 2017;61(11):e00857–17.
Huang JX, Blaskovich MA, Pelingon R, Ramu S, Kavanagh A, Elliott AG, et al. Mucin binding reduces colistin antimicrobial activity. Antimicrob Agents Chemother. 2015;59(10):5925–31.
Hinderling PH. Red blood cells: a neglected compartment in pharmacokinetics and pharmacodynamics. Pharmacol Rev. 1997;49(3):279–95.
Azad MA, Huang JX, Cooper MA, Roberts KD, Thompson PE, Nation RL, et al. Structure–activity relationships for the binding of polymyxins with human α-1-acid glycoprotein. Biochem Pharmacol. 2012;84(3):278–91.
Bouchene S, Marchand S, Friberg LE, Björkman S, Couet W, Karlsson MO, editors. Whole body physiologically-based pharmacokinetic model for colistin and Colistimethate sodium (CMS) in six different species: mouse, rat, rabbit, baboon, pig and human. J Pharmacokinet Pharmacodyn 2013;40(S1):S115–S6
Holford NH, Anderson BJ. Allometric size: the scientific theory and extension to normal fat mass. Eur J Pharm Sci. 2017;109S:S59–S64.
Yousef JM, Chen G, Hill PA, Nation RL, Li J. Melatonin attenuates colistin-induced nephrotoxicity in rats. Antimicrob Agents Chemother. 2011;55(9):4044–9.
Zavascki AP, Nation RL. Nephrotoxicity of polymyxins: is there any difference between colistimethate and polymyxin B? Antimicrob Agents Chemother. 2017;61(3):e02319–16
Manchandani P, Zhou J, Babic JT, Ledesma KR, Truong LD, The TVH. Role of renal drug exposure in polymyxin B-induced nephrotoxicity. Antimicrob Agents Chemother. 2017;61(4):02391–16.
Vardakas KZ, Falagas ME. Colistin versus polymyxin B for the treatment of patients with multidrug-resistant gram-negative infections: a systematic review and meta-analysis. Int J Antimicrob Agents. 2017;49(2):233–8
Ordooei Javan A, Shokouhi S, Sahraei Z. A review on colistin nephrotoxicity. Eur J Clin Pharmacol. 2015;71(7):801–10.
Li M, Gehring R, Riviere JE, Development LZ. Application of a population physiologically based pharmacokinetic model for penicillin G in swine and cattle for food safety assessment. Food Chem Toxicol. 2017;107:74–87.
EMA. Updated advice on the use of colistin products in animals within the European Union: development of resistance and possible impact on human and animal health. European Medicines Agency (EMA), 2016 EMA/CVMP/CHMP/231573/2016.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Figure S1
Goodness-of-fit plots for model validation. Population predicted (PRED) versus observed concentrations or quantities (DV) in log-log scale (A) and linear scale (B). Individual predicted (PRED) versus observed concentrations or quantities (DV) in log-log scale (C) and linear scale (D). (GIF 70 kb)
Figure S2
Visual Predictive Checks of the PBPK model for colistin tissue data in liver (A), muscles (B), skin (C), fat (D), used for model validation. Observed data come from an independent experiment (n°5: 50,000 UI/kg of CMS divided in two IM injection per day during 3 days) that was not used for model calibration. Blue dots represent the observed tissue concentrations; highlighted with grey are the areas between the 5th and 95th percentiles of model simulations, whereas the black solid line represents the median; the purple area represents the 95% confidence interval around the median; the horizontal dashed black line represents the LOQ. In the lower panels, blue areas represent the simulation-based 95% confidence intervals for the fraction of data below the LOQ (BLOQ), whereas the blue solid line represents the actual observed fraction of BLOQ samples. (GIF 138 kb)
Figure S3
Visual Predictive Checks of the PBPK model for CMS tissue data in liver (A), muscles (B), skin (C), fat (D), used for model validation. Observed data come from an independent experiment (n°5: 50,000 UI/kg of CMS divided in two IM injection per day during 3 days) that was not used for model calibration. Blue dots represent the observed tissue concentrations; highlighted with grey are the areas between the 5th and 95th percentiles of model simulations, whereas the black solid line represents the median; the purple area represents the 95% confidence interval around the median; the horizontal dashed black line represents the limit of quantification. In the lower panels, blue areas represent the simulation-based 95% confidence intervals for the fraction of data below the LOQ (BLOQ), whereas the blue solid line represents the actual observed fraction of BLOQ samples. (GIF 94 kb)
Figure S4
Relative contribution of CMS and colistin in total kidney concentrations. CMS concentrations (green), colistin concentrations (red) and total concentrations in kidney after one IV of CMS (10 mg/kg) for a 50-kg pig. (GIF 23 kb)
Figure S5
Evolution of the mass balance predicted by the model after one IV of CMS, as expressed in relative quantities for CMS (A) and colistin (B) in each compartment. GIT: gastro-intestinal tract (GIF 73 kb)
Figure S6
Withdrawal period estimation in a 100-kg pig. Model simulation in kidney after 3 consecutive days of CMS IM injections (50,000 UI/kg of CMS divided in two injections per day) for 1000 virtual pigs of 100 kg. The grey area includes the 1st and 99th percentiles of model simulations, whereas the black solid line represents the median; the horizontal dashed black line represents the kidney MRL (0.20 μg/g). WP: withdrawal period, rounded to the next whole day (GIF 80 kb)
ESM 1
(TXT 28 kb)
Rights and permissions
About this article
Cite this article
Viel, A., Henri, J., Bouchène, S. et al. A Population WB-PBPK Model of Colistin and its Prodrug CMS in Pigs: Focus on the Renal Distribution and Excretion. Pharm Res 35, 92 (2018). https://doi.org/10.1007/s11095-018-2379-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2379-4