Abstract
Purpose
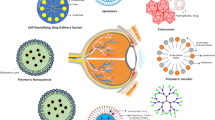
Poor corneal permeability, nasolacrimal drainage and requirement of chronic administration are major drawbacks of existing therapies for ocular inflammation. Hence, we designed topical micelles of PEG2000 conjugated with cholecalciferol (PEGCCF).
Methods
Integrin targeted tacrolimus loaded PEGCCF micelles (TTM) were prepared by solvent diffusion evaporation method and characterized for particle size, osmolality, encapsulation efficiency and drug loading. Therapeutic potential of TTM was evaluated in benzalkonium chloride induced ocular inflammation model in BALB/c mice. Corneal flourescein staining and histopathological analysis of corneal sections was performed.
Results
TTM had a particle size of 45.3 ± 5.3 nm, encapsulation efficiency (88.7 ± 0.9%w/w) and osmolality of 292–296 mOsmol/Kg. TTM significantly reduced the corneal fluorescence as compared to tacrolimus suspension (TACS). H&E staining showed that TTM could restore corneal epithelial thickness, reduce stromal edema (p < 0.05) and decrease number of inflammatory cells (p < 0.01) compared with TACS. Immunohistochemistry analysis demonstrated lower expression of Ki67 + ve cells (p < 0.05) and IL-6 throughout the cornea against TACS (p < 0.01) and the control (p < 0.001).
Conclusions
TTM is an innovative delivery system for improving ocular inflammation due to a) integrin targeting b) PEGCCF in the form of carrier and c) anti-inflammatory and synergistic effect (due to Pgp inhibition) with TAC.






Similar content being viewed by others
Abbreviations
- APC:
-
Antigen presenting cells
- BKC:
-
Benzalkonium chloride
- CRP:
-
C-reactive protein
- DCC:
-
Dicyclohexylcarbodiimide
- DMAP:
-
4-dimethylaminopyridine
- DSPE-PEG2000:
-
1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]
- HCEC:
-
Human corneal epithelial cells
- IFN- γ:
-
Interferon-γ
- IHC:
-
Immunohistochemistry
- iOP:
-
Intraocular pressure
- LC:
-
Langerhans cells
- mPEG2000:
-
Polyethylene glycol methyl ether (average Mn~2000)
- NFAT:
-
Nuclear factor of activated T cells
- PDI:
-
Poly-dispersity index
- PEG:
-
Polyethylene glycol
- PEGCCF:
-
PEG2000 conjugated with cholecalciferol
- Pgp:
-
P-glycoprotein
- RGD:
-
Arg-Gly-Asp
- SF:
-
Sodium fluorescein
- TAC:
-
Tacrolimus
- TACS:
-
Tacrolimus suspension
- TEA:
-
Triethylamine
- TNF-α:
-
Tumor necrosis factor
- TTM:
-
Targeted tacrolimus loaded micelles
- VD3:
-
Cholecalciferol
References
Kymionis GD, Bouzoukis DI, Diakonis VF, Siganos C. Treatment of chronic dry eye: focus on cyclosporine. Clin Ophthalmol (Auckland, NZ). 2008;2(4):829.
Thakur A, Xue M, Stapleton F, Lloyd A, Wakefield D, Willcox M. Balance of pro-and anti-inflammatory cytokines correlates with outcome of acute experimental Pseudomonas aeruginosa keratitis. Infect Immun. 2002;70(4):2187–97.
Wakefield D, Lloyd A. The role of cytokines in the pathogenesis of inflammatory eye disease. Cytokine. 1992;4(1):1–5.
De Paiva CS, Corrales RM, Villarreal AL, Farley WJ, Li D-Q, Stern ME, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83(3):526–35.
Isawi H, Dhaliwal DK. Corneal melting and perforation in Stevens Johnson syndrome following topical bromfenac use. J Cataract Refract Surg. 2007;33(9):1644–1646.
Dua HS, Attre R. Treatment of post-operative inflammation following cataract surgery: a review. Eur Ophthal Rev. 2012;6(2):98–103.
Dinning W. Steroids and the eye--indications and complications. Postgrad Med J. 1976;52(612):634–8.
Waris A, Nagpal G, Nagpal G, Akhtar N. Tacrolimus in ophthalmology. Indian J Clin Exp Ophthalmol. 2015;1(1):3–6.
Kheirkhah A, Zavareh M, Farzbod F, Mahbod M, Behrouz M. Topical 0.005% tacrolimus eye drop for refractory vernal keratoconjunctivitis. Eye. 2011;25(7):872.
Vichyanond P, Kosrirukvongs P. Use of cyclosporine A and tacrolimus in treatment of vernal keratoconjunctivitis. Curr Allergy Asthma Rep. 2013;13(3):308–14.
Park J-H, Joo C-K, Chung SK. Comparative study of tacrolimus and bevacizumab on corneal neovascularization in rabbits. Cornea. 2015;34(4):449–55.
Fukushima A, Ohashi Y, Ebihara N, Uchio E, Okamoto S, Kumagai N, et al. Therapeutic effects of 0.1% tacrolimus eye drops for refractory allergic ocular diseases with proliferative lesion or corneal involvement. Br J Ophthalmol. 2014; https://doi.org/10.1136/bjophthalmol-2013-304453.
Patel P, Patel H, Panchal S, Mehta T. Formulation strategies for drug delivery of tacrolimus: an overview. Int J Pharm Investig. 2012;2(4):169.
Dey S, Patel J, Anand BS, Jain-Vakkalagadda B, Kaliki P, Pal D, et al. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44(7):2909–18.
Chang C, Bahadduri PM, Polli JE, Swaan PW, Ekins S. Rapid identification of P-glycoprotein substrates and inhibitors. Drug Metab Dispos. 2006;34(12):1976–84.
Johnson JA, Grande JP, Roche PC, Campbell RJ, Kumar R. Immuno-localization of the calcitriol receptor, calbinclin-d28k and the plasma membrane calcium pump in the human eye. Curr Eye Res. 1995;14(2):101–8.
Yin Z, Pintea V, Lin Y, Hammock BD, Watsky MA. Vitamin D enhances corneal epithelial barrier function. Invest Ophthalmol Vis Sci. 2011;52(10):7359–7364.
Suzuki T, Sano Y, Kinoshita S. Effects of 1α, 25-dihydroxyvitamin D3 on Langerhans cell migration and corneal neovascularization in mice. Invest Ophthalmo Vis Sci. 2000;41(1):154–158.
Dang S, Lu X, Zhou J, Bai L. Effects of 1alpha, 25-dihydroxyvitamin D3 on the acute immune rejection and corneal neovascularization in high-risk penetrating keratoplasty in rats. Di 1 jun yi da xue xue bao= Academic journal of the first medical college of PLA. 2004;24(8):892–6. 903
Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420(1):1–10.
Kasimova AO, Pavan GM, Danani A, Mondon K, Cristiani A, Scapozza L, et al. Validation of a novel molecular dynamics simulation approach for lipophilic drug incorporation into polymer micelles. J Phys Chem B. 2012;116(14):4338–45.
Tomoda K, Terashima H, Suzuki K, Inagi T, Terada H, Makino K. Enhanced transdermal delivery of indomethacin using combination of PLGA nanoparticles and iontophoresis in vivo. Colloids Surf B: Biointerfaces. 2012;92:50–4.
Bucolo C, Drago F, Salomone S. Ocular drug delivery: a clue from nanotechnology. Front Pharmacol. 2012;3
Weng Y, Liu J, Jin S, Guo W, Liang X, Hu Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm Sin B. 2017;7(3):281–91.
Mun EA, Morrison PWJ, Williams AC, Khutoryanskiy VV. On the Barrier Properties of the Cornea: A Microscopy Study of the Penetration of Fluorescently Labeled Nanoparticles, Polymers, and Sodium Fluorescein. Mol Pharm. 2014;11(10):3556–64.
Lauweryns B, van den Oord JJ, Volpes R, Foets B, Missotten L. Distribution of very late activation integrins in the human cornea. An immunohistochemical study using monoclonal antibodies. Invest Ophthalmol Vis Sci. 1991;32(7):2079–2085.
Stepp MA. Corneal integrins and their functions. Exp Eye Res. 2006;83(1):3–15.
Chu Y, Chen N, Yu H, Mu H, He B, Hua H, et al. Topical ocular delivery to laser-induced choroidal neovascularization by dual internalizing RGD and TAT peptide-modified nanoparticles. Int J Nanomedicine. 2017;12:1353–68.
Singh SR, Grossniklaus HE, Kang SJ, Edelhauser HF, Ambati BK, Kompella UB. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009;16(5):645–59.
Kutlehria S, Behl G, Patel K, Doddapaneni R, Vhora I, Chowdhury N, et al. Cholecalciferol-PEG conjugate based micelles of doxorubicin for treatment of triple-negative breast cancer. AAPS Pharm Sci Tech. 2017;
Lin Z, Liu X, Zhou T, Wang Y, Bai L, He H, et al. A mouse dry eye model induced by topical administration of benzalkonium chloride; 2011. 257–264 p.
Taravati P, Lam DL, Leveque T, Van Gelder RN. Postcataract surgical inflammation. Curr Opin Ophthalmol 2012;23(1):12–18.
Kymionis GD, Bouzoukis DI, Diakonis VF, Siganos C. Treatment of chronic dry eye: focus on cyclosporine. Clin Ophthalmol. 2008;2(4):829–36.
Dey S, Gunda S, Mitra AK. Pharmacokinetics of Erythromycin in Rabbit Corneas after Single-Dose Infusion: Role of P-Glycoprotein as a Barrier to in Vivo Ocular Drug Absorption. J Pharmacol Exp Ther. 2004;311(1):246–55.
Kawazu K, Yamada K, Nakamura M, Ota A. Characterization of Cyclosporin A Transport in Cultured Rabbit Corneal Epithelial Cells: P-Glycoprotein Transport Activity and Binding to Cyclophilin. Invest Ophthalmol Vis Sci. 1999;40(8):1738–1744.
Saha P, Yang JJ, Lee VH. Existence of a p-glycoprotein drug efflux pump in cultured rabbit conjunctival epithelial cells. Invest Ophthalmol Vis Sci. 1998;39(7):1221–1226.
Xue M-L, Zhu H, Thakur A, Willcox M. 1α, 25-Dihydroxyvitamin D3 inhibits pro-inflammatory cytokine and chemokine expression in human corneal epithelial cells colonized with Pseudomonas aeruginosa. Immunol Cell Biol. 2002;80(4):340–5.
Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based Therapeutics: Biological Basis, Clinical Use and New Drugs. Nat Rev Drug Discov. 2016;15(3):173–83.
Kunath K, Merdan T, Hegener O, Häberlein H, Kissel T. Integrin targeting using RGD-PEI conjugates for in vitro gene transfer. J Gene Med. 2003;5(7):588–99.
Barot M, Bagui M, Gokulgandhi MR, Mitra AK. Prodrug Strategies in Ocular Drug Delivery. Medicinal chemistry (Shariqah (United Arab Emirates)). 2012;8(4):753–68.
Duvvuri S, Majumdar S, Mitra AK. Role of metabolism in ocular drug delivery. Curr Drug Metab. 2004;5(6):507–15.
Foroutan SM, Watson DG. Synthesis and characterisation of polyethylene glycol conjugates of hydrocortisone as potential prodrugs for ocular steroid delivery. Int J Pharm. 1997;157(1):103–11.
Foroutan SM, Watson DG. The in vitro evaluation of polyethylene glycol esters of hydrocortisone 21-succinate as ocular prodrugs. Int J Pharm. 1999;182(1):79–92.
Hao T, Chen D, Liu K, Qi Y, Tian Y, Sun P, et al. Micelles of d-α-Tocopheryl Polyethylene Glycol 2000 Succinate (TPGS 2K) for Doxorubicin Delivery with Reversal of Multidrug Resistance. ACS Appl Mater Interfaces. 2015;7(32):18064–75.
Dintaman JM, Silverman JA. Inhibition of P-glycoprotein by D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS). Pharm Res. 1999;16(10):1550–6.
Epstein SP, Chen D, Asbell PA. Evaluation of biomarkers of inflammation in response to benzalkonium chloride on corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther: Offic J Assoc Ocul Pharmacol Ther. 2009;25(5):415–24.
Warcoin E, Clouzeau C, Roubeix C, Raveu AL, Godefroy D, Riancho L, et al. Hyperosmolarity and Benzalkonium Chloride Differently Stimulate Inflammatory Markers in Conjunctiva-Derived Epithelial Cells in vitro. Ophthalmic Res. 2017;58(1):40–8.
Thomson AW, Bonham CA, Zeevi A. Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit. 1995;17(6):584–91.
Lin Z, Liu X, Zhou T, Wang Y, Bai L, He H, et al. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol Vis. 2011;17:257–64.
De Saint Jean M, Brignole F, Bringuier AF, Bauchet A, Feldmann G, Baudouin C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999;40(3):619–630.
Li C, Song Y, Luan S, Wan P, Li N, Tang J, et al. Research on the Stability of a Rabbit Dry Eye Model Induced by Topical Application of the Preservative Benzalkonium Chloride. PLoS One. 2012;7(3):e33688.
Ebihara N, Ohtomo K, Tokura T, Ushio H, Murakami A. Effect of tacrolimus on chemokine production by corneal myofibroblasts via toll-like receptors, compared with cyclosporine and dexamethasone. Cornea. 2011;30(6):702–8.
Dickie LJ, Church LD, Coulthard LR, Mathews RJ, Emery P, McDermott MF. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology (Oxford, England). 2010;49(8):1466–71.
Carvalho JTG, Schneider M, Cuppari L, Grabulosa CC, Aoike TD, QBM R, et al. Cholecalciferol decreases inflammation and improves vitamin D regulatory enzymes in lymphocytes in the uremic environment: A randomized controlled pilot trial. PLos One. 2017;12(6):e0179540.
Brumback RA, Leech RW. Neuropathology and basic neuroscience: Springer Science & Business Media; 2012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kutlehria, S., Vhora, I., Bagde, A. et al. Tacrolimus Loaded PEG-Cholecalciferol Based Micelles for Treatment of Ocular Inflammation. Pharm Res 35, 117 (2018). https://doi.org/10.1007/s11095-018-2376-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2376-7