Abstract
Purpose
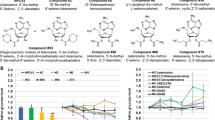
A series of novel polycyclic aromatic compounds that augment the rate of glucose uptake in L6 myotubes and increase glucose-stimulated insulin secretion from beta-cells were synthesized. Designing these molecules, we have aimed at the two main pathogenic mechanisms of T2D, deficient insulin secretion and diminished glucose clearance. The ultimate purpose of this work was to create a novel antidiabetic drug candidate with bi-functional mode of action.
Methods
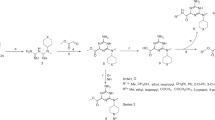
All presented compounds were synthesized, and characterized in house. INS-1E cells and L6 myoblasts were used for the experiments. The rate of glucose uptake, mechanism of action, level of insulin secretion and the druggability of the lead compound were studied.
Results
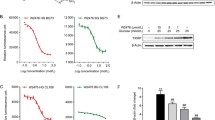
The lead compound (6-(1,3-dithiepan-2-yl)-2-phenylchromane), dose- and time-dependently at the low μM range increased the rate of glucose uptake in L6 myotubes and insulin secretion in INS-1E cells. The compound exerted its effects through the activation of the LKB1 (Liver Kinase B1)-AMPK pathway. In vitro metabolic parameters of this lead compound exhibited good druggability.
Conclusions
We anticipate that bi-functionality (increased rate of glucose uptake and augmented insulin secretion) will allow the lead compound to be a starting point for the development of a novel class of antidiabetic drugs.









Similar content being viewed by others
Abbreviations
- [3H]dGlc:
-
1-Tritium-2-deoxyglucose
- ACC:
-
Acetyl-CoA carboxylase
- AICAR:
-
5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside
- Akt/PKB:
-
Akt/Protein kinase B
- AMPK:
-
AMP kinase
- AS160 :
-
AMPK substrate of phosphorylation 160 kDA
- BDA :
-
Benzaldehyde dimethyl acetal
- CYP :
-
Cytochrome P450
- DCM:
-
Dichloromethane
- DEAD:
-
Diethyl azodicarboxylate
- DMF:
-
Dimethrylformamide
- GLUT4:
-
Glucose transporter type 4
- GSIS:
-
Glucose-stimulated insulin secretion
- hERG:
-
“Human ether-à-go-go”-related gene channels
- HRP :
-
Horseradish peroxidase
- LKB1:
-
Liver Kinase B1
- mGPD:
-
Mitochondrial glycerophosphate dehydrogenase
- OPD:
-
O-phenylenediamine
- PCC:
-
Pyridinium Chlorochromate
- PMSF:
-
Phenylmethanesulfonylfluoride
- QTof:
-
Quadrupole time of-flight mass spectrometer
- T2D:
-
Type 2 diabetes
- THF:
-
Tetrahydrofuran
- TMS:
-
Tetramethylsilane
- TMSCl:
-
Trimethylsilyl chloride
- TosCl:
-
Toluenesulfonyl chloride
References
Rojasand LB, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr. 2013;5:6.
Consoli A, Gomis R, Halimi S, Home PD, Mehnert H, Strojek K, et al. Initiating oral glucose-lowering therapy with metformin in type 2 diabetic patients: an evidence-based strategy to reduce the burden of late-developing diabetes complications. Diabetes Metab. 2004;30:509–16.
Jensenand TE, Richter EA. Regulation of glucose and glycogen metabolism during and after exercise. J Physiol. 2012;590:1069–76.
Rana S, Blowers EC, Natarajan A. Small molecule adenosine 5′-monophosphate activated protein kinase (AMPK) modulators and human diseases. J Med Chem. 2015;58:2–29.
Karlssonand HK, Zierath JR. Insulin signaling and glucose transport in insulin resistant human skeletal muscle. Cell Biochem Biophys. 2007;48:103–13.
Richterand EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993–1017.
Klip A, Schertzer JD, Bilan PJ, Thong F, Antonescu C. Regulation of glucose transporter 4 traffic by energy deprivation from mitochondrial compromise. Acta Physiol (Oxf). 2009;196:27–35.
Ojuka EO. Role of calcium and AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. Proc Nutr Soc. 2004;63:275–8.
Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. 2015;33:1–7.
Kyriakis JM. At the crossroads: AMP-activated kinase and the LKB1 tumor suppressor link cell proliferation to metabolic regulation. J Biol. 2003;2:26.
Jiang SJ, Dong H, Li JB, Xu LJ, Zou X, Wang KF, et al. Berberine inhibits hepatic gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced diabetic rats. World J Gastroenterol. 2015;21:7777–85.
Xie Z, Ding SQ, Shen YF. Silibinin activates AMP-activated protein kinase to protect neuronal cells from oxygen and glucose deprivation-re-oxygenation. Biochem Biophys Res Commun. 2014;454:313–9.
Yang Y, Zhao Z, Liu Y, Kang X, Zhang H, Meng M. Suppression of oxidative stress and improvement of liver functions in mice by ursolic acid via LKB1-AMP-activated protein kinase signaling. J Gastroenterol Hepatol. 2015;30:609–18.
Zaks I, Getter T, Gruzman A. Activators of AMPK: not just for Type II diabetes. Future Med Chem. 2014;6:1325–53.
Cameronand KO, Kurumbail RG. Recent progress in the identification of adenosine monophosphate-activated protein kinase (AMPK) activators. Bioorg Med Chem Lett. 2016;26:5139–48.
Giordanettoand F, Karis D. Direct AMP-activated protein kinase activators: a review of evidence from the patent literature. Expert Opin Ther Pat. 2012;22:1467–77.
Sliwinskaand A, Drzewoski J. Molecular action of metformin in hepatocytes: an updated insight. Curr Diabetes Rev. 2015;11:175–81.
Zhou JY, Xu B, Li L, New Role A. for an Old Drug: Metformin Targets MicroRNAs in Treating Diabetes and Cancer. Drug Dev Res. 2015;76:263–9.
Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953–66.
Cohen G, Riahi Y, Alpert E, Gruzman A, Sasson S. The roles of hyperglycaemia and oxidative stress in the rise and collapse of the natural protective mechanism against vascular endothelial cell dysfunction in diabetes. Arch Physiol Biochem. 2007;113:259–67.
Cameron KO, Kung DW, Kalgutkar AS, Kurumbail RG, Miller R, Salatto CT, et al. Discovery and Preclinical Characterization of 6-Chloro-5-[4-(1-hydroxycyclobutyl)phenyl]-1H-indole-3-carboxylic Acid (PF-06409577), a Direct Activator of Adenosine Monophosphate-activated Protein Kinase (AMPK), for the Potential Treatment of Diabetic Nephropathy. J Med Chem. 2016;59:8068–81.
Hsu MH, Savas U, Lasker JM, Johnson EF. Genistein, resveratrol, and 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside induce cytochrome P450 4F2 expression through an AMP-activated protein kinase-dependent pathway. J Pharmacol Exp Ther. 2011;337:125–36.
Benziane B, Bjornholm M, Lantier L, Viollet B, Zierath JR, Chibalin AV. AMP-activated protein kinase activator A-769662 is an inhibitor of the Na(+)-K(+)-ATPase. Am J Physiol Cell Physiol. 2009;297:C1554–66.
Mirguet O, Sautet S, Clement CA, Toum J, Donche F, Marques C, et al. Discovery of Pyridones As Oral AMPK Direct Activators. ACS Med Chem Lett. 2013;4:632–6.
Ojukaand EO, Goyaram V. Mechanisms in exercise-induced increase in glucose disposal in skeletal muscle. Med Sport Sci. 2014;60:71–81.
Krishan S, Richardson DR, Sahni S. Adenosine monophosphate-activated kinase and its key role in catabolism: structure, regulation, biological activity, and pharmacological activation. Mol Pharmacol. 2015;87:363–77.
Miglianico M, Nicolaes GA, Neumann D. Pharmacological Targeting of AMP-Activated Protein Kinase and Opportunities for Computer-Aided Drug Design. J Med Chem. 2016;59:2879–93.
Kone M, Pullen TJ, Sun G, Ibberson M, Martinez-Sanchez A, Sayers S, et al. LKB1 and AMPK differentially regulate pancreatic beta-cell identity. FASEB J. 2014;28:4972–85.
Swisa A, Granot Z, Tamarina N, Sayers S, Bardeesy N, Philipson L, et al. Loss of Liver Kinase B1 (LKB1) in Beta Cells Enhances Glucose-stimulated Insulin Secretion Despite Profound Mitochondrial Defects. J Biol Chem. 2015;290:20934–46.
Sun G, Tarasov AI, McGinty JA, French PM, McDonald A, Leclerc I, et al. LKB1 deletion with the RIP2.Cre transgene modifies pancreatic beta-cell morphology and enhances insulin secretion in vivo. Am J Physiol Endocrinol Metab. 2010;298:E1261–73.
Fu A, Robitaille K, Faubert B, Reeks C, Dai XQ, Hardy AB, et al. LKB1 couples glucose metabolism to insulin secretion in mice. Diabetologia. 2015;58:1513–22.
Granot Z, Swisa A, Magenheim J, Stolovich-Rain M, Fujimoto W, Manduchi E, et al. LKB1 regulates pancreatic beta cell size, polarity, and function. Cell Metab. 2009;10:296–308.
Fu A, Ng AC, Depatie C, Wijesekara N, He Y, Wang GS, et al. Loss of Lkb1 in adult beta cells increases beta cell mass and enhances glucose tolerance in mice. Cell Metab. 2009;10:285–95.
Fu A, Eberhard CE, Screaton RA. Role of AMPK in pancreatic beta cell function. Mol Cell Endocrinol. 2013;366:127–34.
Langelueddecke C, Jakab M, Ketterl N, Lehner L, Hufnagl C, Schmidt S, et al. Effect of the AMP-kinase modulators AICAR, metformin and compound C on insulin secretion of INS-1E rat insulinoma cells under standard cell culture conditions. Cell Physiol Biochem. 2012;29:75–86.
da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J. 2003;371:761–74.
Sun G, Tarasov AI, McGinty J, McDonald A, da Silva Xavier G, Gorman T, et al. Ablation of AMP-activated protein kinase alpha1 and alpha2 from mouse pancreatic beta cells and RIP2.Cre neurons suppresses insulin release in vivo. Diabetologia. 2010;53:924–36.
Ryu GR, Lee MK, Lee E, Ko SH, Ahn YB, Kim JW, et al. Activation of AMP-activated protein kinase mediates acute and severe hypoxic injury to pancreatic beta cells. Biochem Biophys Res Commun. 2009;386:356–62.
Beall C, Watterson KR, McCrimmon RJ, Ashford ML. AMPK modulates glucose-sensing in insulin-secreting cells by altered phosphotransfer to KATP channels. J Bioenerg Biomembr. 2013;45:229–41.
Matsuda T, Takai T, Suzuki E, Kanno A, Koyanagi-Kimura M, Asahara S-i, et al. Regulation of Pancreatic β Cell Mass by Cross-Interaction between CCAAT Enhancer Binding Protein β Induced by Endoplasmic Reticulum Stress and AMP-Activated Protein Kinase Activity. PLoS One. 2015;10:e0130757.
Wang K, Sun Y, Lin P, Song J, Zhao R, Li W, et al. Liraglutide Activates AMPK Signaling and Partially Restores Normal Circadian Rhythm and Insulin Secretion in Pancreatic Islets in Diabetic Mice. Biol Pharm Bull. 2015;38:1142–9.
Pasternak L, Meltzer-Mats E, Babai-Shani G, Cohen G, Viskind O, Eckel J, et al. Benzothiazole derivatives augment glucose uptake in skeletal muscle cells and stimulate insulin secretion from pancreatic beta-cells via AMPK activation. Chem Commun (Camb). 2014;50:11222–5.
Beall C, Piipari K, Al-Qassab H, Smith MA, Parker N, Carling D, et al. Loss of AMP-activated protein kinase α2 subunit in mouse β-cells impairs glucose-stimulated insulin secretion and inhibits their sensitivity to hypoglycaemia. Biochem J. 2010;429:323–33.
Gruzman A, Elgart A, Viskind O, Billauer H, Dotan S, Cohen G, et al. Antihyperglycaemic activity of 2,4:3,5-dibenzylidene-D-xylose-diethyl dithioacetal in diabetic mice. J Cell Mol Med. 2012;16:594–604.
Gruzman A, Shamni O, Ben Yakir M, Sandovski D, Elgart A, Alpert E, et al. Novel D-Xylose Derivatives Stimulate Muscle Glucose Uptake by Activating AMP-Activated Protein Kinase α. J Med Chem. 2008;51:8096–108.
Meltzer-Mats E, Babai-Shani G, Pasternak L, Uritsky N, Getter T, Viskind O, et al. Synthesis and mechanism of hypoglycemic activity of benzothiazole derivatives. J Med Chem. 2013;56:5335–50.
Gottlieb HE, Kotlyar V, Nudelman A. NMR chemical shifts of common laboratory solvents as trace impurities. J Org Chem. 1997;62:7512–5.
La Regina G, Diodata D'Auria F, Tafi A, Piscitelli F, Olla S, Caporuscio F, et al. 1-[(3-Aryloxy-3-aryl)propyl]-1H-imidazoles, New Imidazoles with Potent Activity against Candida albicans and Dermatophytes. Synthesis, Structure-Activity Relationship, and Molecular Modeling Studies. J Med Chem. 2008;51:3841–55.
Hodgetts KJ. A stereocontrolled route to 2-substituted chromans. Tetrahedron Lett. 2000;41:8655–9.
Hodgetts KJ. Inter- and intramolecular Mitsunobu reaction based approaches to 2-substituted chromans and chroman-4-ones. Tetrahedron. 2005;61:6860–70.
Ongand BS, Chan TH. A simple method of dithioacetalization and dithioketalization. Synth Commun. 1977;7:283–6.
Kimura M, Hamakawa T, Hanabusa K, Shirai H, Kobayashi N. Synthesis of Multicomponent Systems Composed of One Phthalocyanine and Four Terpyridine Ligands. Inorg Chem. 2001;40:4775–9.
Handrickand GR, Atkinson ER. Potential antiradiation drugs. III. 2-Amino-2-alkyl-1,3-propanedithiols and 3-amino-4-mercapto-1-butanol. J Med Chem. 1966;9:558–62.
Zhong W, Tang Y, Zampella G, Wang X, Yang X, Hu B, et al. A rare bond between a soft metal (FeI) and a relatively hard base (RO-, R = phenolic moiety). Inorg Chem Commun. 2010;13:1089–92.
Munder A, Moskovitz Y, Redko B, Levy AR, Ruthstein S, Gellerman G, et al. Antiproliferative Effect of Novel Aminoacridine-based Compounds. Med Chem. 2015;11:373–82.
Shimanovich U, Munder A, Azoia NG, Cavaco-Paulo A, Gruzman A, Knowles TP, et al. Sonochemically-induced spectral shift as a probe of green fluorescent protein release from nano capsules. RSC Adv. 2014;4:10303–9.
Suckow AT, Zhang C, Egodage S, Comoletti D, Taylor P, Miller MT, et al. Transcellular neuroligin-2 interactions enhance insulin secretion and are integral to pancreatic beta cell function. J Biol Chem. 2012;287:19816–26.
Sanderson RJ, Shepperdson RT, Vatter AE, Talmage DW. Isolation and enumeration of peripheral blood monocytes. J Immunol. 1977;118:1409–14.
Eckshtain-Levi M, Lavi R, Yufit DS, Daniel B, Green O, Fleker O, et al. A versatile water-soluble chelating and radical scavenging platform. Chem Commun (Camb). 2016;52:2350–3.
Shapira R, Rudnick S, Daniel B, Viskind O, Aisha V, Richman M, et al. Multifunctional cyclic D,L-α-peptide architectures stimulate non-insulin dependent glucose uptake in skeletal muscle cells and protect them against oxidative stress. J Med Chem. 2013;56:6709–18.
Zatara G, Hertz R, Shaked M, Mayorek N, Morad E, Grad E, et al. Suppression of FoxO1 activity by long-chain fatty acyl analogs. Diabetes. 2011;60:1872–81.
Cohen G, Riahi Y, Shamni O, Guichardant M, Chatgilialoglu C, Ferreri C, et al. Role of lipid peroxidation and PPAR-delta in amplifying glucose-stimulated insulin secretion. Diabetes. 2011;60:2830–42.
Li XQ, Andersson TB, Ahlstrom M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32:821–7.
Niwa T, Shiraga T, Takagi A. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol Pharm Bull. 2005;28:1805–8.
Mori T, Sawada Y, Oku A. Ring-expansion of thioacetal ring via bicyclosulfonium ylide. Effect Of protic nucleophile on ylide intermediate J Org Chem. 2000;65:3620–5.
Cui Z, Chen X, Lu B, Park SK, Xu T, Xie Z, et al. Preliminary quantitative profile of differential protein expression between rat L6 myoblasts and myotubes by stable isotope labeling with amino acids in cell culture. Proteomics. 2009;9:1274–92.
Wertheimer E, Sasson S, Cerasi E. Regulation of hexose transport in L8 myocytes by glucose: possible sites of interaction. J Cell Physiol. 1990;143:330–6.
Itani SI, Saha AK, Kurowski TG, Coffin HR, Tornheim K, Ruderman NB. Glucose autoregulates its uptake in skeletal muscle: involvement of AMP-activated protein kinase. Diabetes. 2003;52:1635–40.
Ben-Yakir M, Gruzman A, Alpert E, Sasson S. Glucose transport regulators. Current Medicinal Chemistry-Immunology. Endocr Metab Agents. 2005;5:519–27.
Shamni O, Cohen G, Gruzman A, Zaid H, Klip A, Cerasi E, et al. Regulation of GLUT4 activity in myotubes by 3-O-methyl-d-glucose. Biochim Biophys Acta. 2017;1859:1900–10.
Shamni O, Cohen G, Gruzman A, Zaid H, Klip A, Cerasi E, et al. Supportive data on the regulation of GLUT4 activity by 3-O-methyl-D-glucose. Data Brief. 2017;14:329–36.
Furtado LM, Somwar R, Sweeney G, Niu W, Klip A. Activation of the glucose transporter GLUT4 by insulin. Biochem Cell Biol. 2002;80:569–78.
Chiu TT, Jensen TE, Sylow L, Richter EA, Klip A. Rac1 signalling towards GLUT4/glucose uptake in skeletal muscle. Cell Signal. 2011;23:1546–54.
Kottakisand F, Bardeesy N. LKB1-AMPK axis revisited. Cell Res. 2012;22:1617–20.
Cao S, Li B, Yi X, Chang B, Zhu B, Lian Z, et al. Effects of exercise on AMPK signaling and downstream components to PI3K in rat with type 2 diabetes. PLoS One. 2012;7:e51709.
Deshmukh AS, Hawley JA, Zierath JR. Exercise-induced phospho-proteins in skeletal muscle. Int J Obes (Lond) 32 Suppl. 2008;4:S18–23.
Hardieand DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002;30:1064–70.
Ross FA, MacKintosh C, Hardie DG. AMP-activated protein kinase: a cellular energy sensor that comes in 12 flavours. FEBS J. 2016;283:2987–3001.
Mackenzieand RW, Elliott BT. Akt/PKB activation and insulin signaling: a novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab Syndr Obes. 2014;7:55–64.
Thomas D, Karle CA, Kiehn J. The cardiac hERG/IKr potassium channel as pharmacological target: structure, function, regulation, and clinical applications. Curr Pharm Des. 2006;12:2271–83.
Pierson JB, Berridge BR, Brooks MB, Dreher K, Koerner J, Schultze AE, et al. A public-private consortium advances cardiac safety evaluation: achievements of the HESI Cardiac Safety Technical Committee. J Pharmacol Toxicol Methods. 2013;68:7–12.
Zangerand UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–41.
Sager JE, Lutz JD, Foti RS, Davis C, Kunze KL, Isoherranen N. Fluoxetine- and norfluoxetine-mediated complex drug-drug interactions: in vitro to in vivo correlation of effects on CYP2D6, CYP2C19, and CYP3A4. Clin Pharmacol Ther. 2014;95:653–62.
Quinn DI, Nemunaitis J, Fuloria J, Britten CD, Gabrail N, Yee L, et al. Effect of the cytochrome P450 2C19 inhibitor omeprazole on the pharmacokinetics and safety profile of bortezomib in patients with advanced solid tumours, non-Hodgkin's lymphoma or multiple myeloma. Clin Pharmacokinet. 2009;48:199–209.
Furuta S, Kamada E, Suzuki T, Sugimoto T, Kawabata Y, Shinozaki Y, et al. Inhibition of drug metabolism in human liver microsomes by nizatidine, cimetidine and omeprazole. Xenobiotica. 2001;31:1–10.
Wen X, Wang J-S, Neuvonen PJ, Backman JT. Isoniazid is a mechanism-based inhibitor of cytochrome P450 1A2, 2A6, 2C19 and 3A4 isoforms in human liver microsomes. Eur J Clin Pharmacol. 2002;57:799–804.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOC 54625 kb)
Rights and permissions
About this article
Cite this article
Rozentul, N., Avrahami, Y., Shubely, M. et al. A Novel Phenylchromane Derivative Increases the Rate of Glucose Uptake in L6 Myotubes and Augments Insulin Secretion from Pancreatic Beta-Cells by Activating AMPK. Pharm Res 34, 2873–2890 (2017). https://doi.org/10.1007/s11095-017-2271-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2271-7