Abstract
Purpose
To develop and validate a Level A in vitro-in vivo correlation (IVIVC) for potassium chloride extended-release (ER) formulations.
Methods
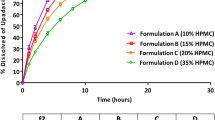
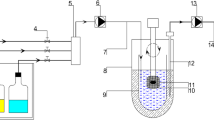
Three prototype ER formulations of potassium chloride with different in vitro release rates were developed and their urinary pharmacokinetic profiles were evaluated in healthy subjects. A mathematical model between in vitro dissolution and in vivo urinary excretion, a surrogate for measuring in vivo absorption, was developed using time-scale and time-shift parameters. The IVIVC model was then validated based on internal and external predictability.
Results
With the established IVIVC model, there was a good correlation between the observed fraction of dose excreted in urine and the time-scaled and time-shifted fraction of the drug dissolved, and between the in vitro dissolution time and the in vivo urinary excretion time for the ER formulations. The percent prediction error (%PE) on cumulative urinary excretion over the 24 h interval (Ae0–24h) and maximum urinary excretion rate (Rmax) was less than 15% for the individual formulations and less than 10% for the average of the two formulations used to develop the model. Further, the %PE values using external predictability were below 10%.
Conclusions
A novel Level A IVIVC was successfully developed and validated for the new potassium chloride ER formulations using urinary pharmacokinetic data. This successful IVIVC may facilitate future development or manufacturing changes to the potassium chloride ER formulation.




Similar content being viewed by others
Abbreviations
- Ae :
-
Amount excreted in each interval
- BE:
-
Bioequivalence
- Ae0–24 :
-
Cumulative urinary excretion over the 24 h interval
- ER:
-
Extended-release
- IVIVC:
-
In vitro-in vivo correlation
- Rmax :
-
Maximum urinary excretion rate
- PE:
-
Prediction error
- Tmax :
-
Time of maximal urinary excretion
References
National Research Council (US) Subcommittee on the tenth edition of the recommended dietary allowances. Water and electrolytes. Recommended dietary allowances. 10th ed. Washington: National Academies Press; 1989.
Ernst ME, Moser M. Use of diuretics in patients with hypertension. N Engl J Med. 2009;361:2153–64.
Ehrlich EN. Aldosterone, the adrenal cortex, and hypertension. Annu Rev Med. 1968;19:373–98.
Schmitz K. Providing the best possible care: an overview of the current understanding of diabetic ketoacidosis. Aust Crit Care. 2000;13:22–7.
Alsop WR, Moore JG, Rollins DE, Tolman KG. The effects of five potassium chloride preparations on the upper gastrointestinal mucosa in healthy subjects receiving glycopyrrolate. J Clin Pharmacol. 1984;24:235–9.
Betlach CJ, Arnold JD, Frost RW, Leese PT, Gonzalez MA. Bioavailability and pharmacokinetics of a new sustained-release potassium chloride tablet. Pharm Res. 1987;4:409–11.
Guidance for Industry. Potassium chloride modified release tablets and capsules: in vivo bioequivalence and in vitro dissolution testing. Food and Drug Administration, Center for Drug Evaluation and Research; 2005.
Emami J. In vitro - in vivo correlation: from theory to applications. J Pharm Pharm Sci. 2006;9:169–89.
Guidance for Industry. Extended release oral dosage forms: development, evaluation, and application of in vitro / in vivo correlations. Food and Drug Administration, Center for Drug Evaluation and Research; 1997.
Qiu Y, Duan J. In vitro–in vivo correlations: fundamentals, development considerations, and applications. In: Developing solid oral dosage forms: pharmaceutical theory and Practice, 2nd ed. San Diego: Academic Press; 2017. p. 415–425.
Qiu Y, Lee P. Rational design of oral modified-release drug delivery systems. In: Developing oral dosage forms: pharmaceutical theory and practice, 2nd ed. San Diego: Academic Press; 2017. p. 519–554.
Giebisch GH, Wang W-H. Potassium transport--an update. J Nephrol. 2010;23(Suppl 16):S97–104.
Rastegar A. Serum potassium. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston: Butterworths; 1990.
Jambhekar SS, Breen PJ. Chapter 6.5.2.Wangner-Nelson method from mass of drug in urine data. Basic Pharmacokinet, 2nd ed. 2009. p. 105–136.
Wagner JG, Nelson E. Kinetic analysis of blood levels and urinary excretion in the absorptive phase after single doses of drug. J Pharm Sci. 1964;53:1392–403.
Benech H, Grognet JM. Recent data on the evaluation of magnesium bioavailability in humans. Magnes Res. 1995;8:277–84.
ACKNOWLEDGMENTS AND DISCLOSURES
This study was sponsored by AbbVie Inc. AbbVie Inc. contributed to the study design; research; data interpretation, writing, review, and approval of the manuscript for publication. Rajendar K Mittapalli, Patrick Marroum, Yihong Qiu, Kathleen Apfelbaum, Hao Xiong are employees of AbbVie Inc. All authors may hold AbbVie stocks or options.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
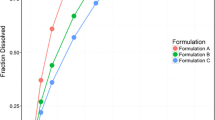
Comparison of observed versus model predicted fraction dissolved for potassium chloride extended release formulations A (Fast), B (Intermediate), and C (Slow). (PNG 36 kb)
Rights and permissions
About this article
Cite this article
Mittapalli, R.K., Marroum, P., Qiu, Y. et al. Development of In Vitro-In Vivo Correlation for Potassium Chloride Extended Release Tablet Formulation Using Urinary Pharmacokinetic Data. Pharm Res 34, 1527–1533 (2017). https://doi.org/10.1007/s11095-017-2179-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2179-2