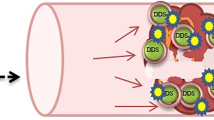
Abstract
Purpose
To test the hypothesis that a mixture combining fast and slower release rate microspheres can restore blood flow rapidly and prevent formation of another blockage in thrombolysis.
Methods
We used polyethylene glycol (PEG) microspheres which provide the release of the encapsulated streptokinase (SK) on the scale of minutes, and Eudragit FS30D (Eud), a polymethacrylate polymer, for development of delayed release microspheres which were desirable to prevent a putative second thrombus. Eud microspheres were coated with chitosan (CS) to further extend half-life. Experiments included the development, characterization of Eud/SK and CS-Eud/SK microspheres, and in vitro thrombolytic studies of the mixtures of PEG/SK and Eud /SK microspheres and of PEG/SK and CS-Eud/SK microspheres.
Results
CS-Eud/SK microspheres have slightly lower encapsulation efficiency, reduced activity of SK, and a much slower release of SK when compared with microspheres of Eud/SK microspheres. Counter-intuitively, slower release leads to faster thrombolysis after reocclusion as a result of greater retention of agent and the mechanism of distributed intraclot thrombolysis.
Conclusions
A mixture of PEG/SK and CS-Eud/SK microspheres could break up the blood clot rapidly while providing clot-lytic efficacy in prevention of a second blockage up to 4 h.










Similar content being viewed by others
Abbreviations
- CS:
-
Chitosan
- EDC:
-
1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide
- EE:
-
Encapsulation efficiency
- Eud:
-
Eudragit FS30D
- FT-IR:
-
Fourier transform infrared
- MES:
-
2-(N-morpholino)ethanesulfonic acid
- NHS:
-
N-hydroxysuccinimide
- PA:
-
Plasminogen activator
- PAI:
-
Plasminogen activator inhibitor
- PBS:
-
Phosphate-buffered saline
- PEG:
-
Polyethylene glycol
- PPP:
-
Platelet-poor plasma
- PRP:
-
Platelet-rich plasma
- PVA:
-
Poly(vinyl alcohol)
- SEM:
-
Scanning electron microscope
- SK:
-
Streptokinase
- TEM:
-
Transmission electron microscope
- tPA:
-
Tissue-type plasminogen activator
References
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation. 2014;129:E28–292.
Vaidya B, Agrawal GP, Vyas SP. Functionalized carriers for the improved delivery of plasminogen activators. Int J Pharm. 2012;424:1–11.
Kunamneni A, Abdelghani TTA, Ellaiah P. Streptokinase—the drug of choice for thrombolytic therapy. J Thromb Thrombolysis. 2007;23:9–23.
Anand S, Kudallur V, Pitman EB, Diamond SL. Mechanisms by which thrombolytic therapy results in nonuniform lysis and residual thrombus after reperfusion. Ann Biomed Eng. 1997;25:964–74.
Gyongyosi M, Wexberg P, Kiss K, Yang P, Sperker W, Sochor H, et al. Adaptive remodeling of the infarct-related artery is associated with recurrent ischemic events after thrombolysis in acute myocardial infarction. Coron Artery Dis. 2001;12:167–72.
Ohman EM, Califf RM, Topol EJ, Candela R, Abbottsmith C, Ellis S, et al. Consequences of reocclusion after successful reperfusion therapy in acute myocardial-infarction. Circulation. 1990;82:781–91.
Wang SS, Chou NK, Chung TW. The t-PA-encapsulated PLGA nanoparticles shelled with CS or CS-GRGD alter both permeation through and dissolving patterns of blood clots compared with t-PA solution: an in vitro thrombolysis study. J Biomed Mater Res A. 2009;91A:753–61.
Vaidya B, Nayak MK, Dash D, Agrawal GP, Vyas SP. Development and characterization of site specific target sensitive liposomes for the delivery of thrombolytic agents. Int J Pharm. 2011;403:254–61.
Leach JK, Patterson E, O’Rear EA. Distributed intraclot thrombolysis: mechanism of accelerated thrombolysis with encapsulated plasminogen activators. J Thromb Haemost. 2004;2:1548–55.
Khoobehi Band Peyman GA. Accelerated thrombolysis and reperfusion in a primate model of branch vein occlusion by liposomal encapsulation of streptokinase. Invest Ophthalmol Vis Sci. 1997;38:4879.
Leach JK, O’Rear EA, Patterson E, Miao P, Johnson AE. Accelerated thrombolysis in a rabbit model of carotid artery thrombosis with liposome-encapsulated and microencapsulated streptokinase. Thromb Haemost. 2003;90:64–70.
Chung TW, Wang SS, Tsai WJ. Accelerating thrombolysis with chitosan-coated plasminogen activators encapsulated in poly-(lactide-co-glycolide) (PLGA) nanoparticles. Biomaterials. 2008;29:228–37.
Mukhametova LI, Aisina RB, Tyupa DV, Medvedeva AS, Gershkovich KB. Properties of streptokinase incorporated into polyethylene glycol microcapsules. Russ J Bioorg Chem. 2013;39:390–6.
Absar S, Nahar K, Kwon YM, Ahsan F. Thrombus-targeted nanocarrier attenuates bleeding complications associated with conventional thrombolytic therapy. Pharm Res. 2013;30:1663–76.
Bi F, Zhang J, Su YJ, Tang YC, Liu JN. Chemical conjugation of urokinase to magnetic nanoparticles for targeted thrombolysis. Biomaterials. 2009;30:5125–30.
Yang HW, Hua MY, Lin KJ, Wey SP, Tsai RY, Wu SY, et al. Bioconjugation of recombinant tissue plasminogen activator to magnetic nanocarriers for targeted thrombolysis. Int J Nanomed. 2012;7:5159–73.
Smith DAB, Vaidya SS, Kopechek JA, Huang S-L, Klegerman ME, McPherson DD, et al. Ultrasound-triggered release of recombinant tissue-type plasminogen activator from echogenic liposomes. Ultrasound Med Biol. 2010;36:145–57.
Uesugi Y, Kawata H, Jo J, Saito Y, Tabata Y. An ultrasound-responsive nano delivery system of tissue-type plasminogen activator for thrombolytic therapy. J Control Release. 2010;147:269–77.
Torno MD, Kaminski MD, Xie Y, Meyers RE, Mertz CJ, Liu X, et al. Improvement of in vitro thrombolysis employing magnetically-guided microspheres. Thromb Res. 2008;121:799–811.
Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, Mammoto T, et al. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science. 2012;337:738–42.
Nguyen PD, Orear EA, Johnson AE, Patterson E, Whitsett TL, Bhakta R. Accelerated thrombolysis and reperfusion in a canine model of myocardial-infarction by liposomal encapsulation of streptokinase. Circ Res. 1990;66:875–8.
Piras AM, Chiellini F, Fiumi C, Bartoli C, Chiellini E, Fiorentino B, et al. A new biocompatible nanoparticle delivery system for the release of fibrinolytic drugs. Int J Pharm. 2008;357:260–71.
Holt B, Sen Gupta A. Streptokinase loading in liposomes for vascular targeted nanomedicine applications: encapsulation efficiency and effects of processing. J Biomater Appl. 2012;26:509–27.
Kim JY, Kim JK, Park JS, Byun Y, Kim CK. The use of PEGylated liposomes to prolong circulation lifetimes of tissue plasminogen activator. Biomaterials. 2009;30:5751–6.
Marder VJ, Sherry S. Thrombolytic therapy: current status. N Engl J Med. 1988;318:1512–20.
Leach JK, Patterson E, O’Rear EA. Encapsulation of a plasminogen activator speeds reperfusion, lessens infarct and reduces blood loss in a canine model of coronary artery thrombosis. Thromb Haemost. 2004;91:1213–8.
Dvorackova K, Rabiskova M, Muselik J, Gajdziok J, Bajerova M. Coated hard capsules as the pH-dependent drug transport systems to ileo-colonic compartment. Drug Dev Ind Pharm. 2011;37:1131–40.
Sinha VR, Singla AK, Wadhawan S, Kaushik R, Kumria R, Bansal K, et al. Chitosan microspheres as a potential carrier for drugs. Int J Pharm. 2004;274:1–33.
Zhang D, Sun P, Li P, Xue A, Zhang X, Zhang H, et al. A magnetic chitosan hydrogel for sustained and prolonged delivery of Bacillus Calmette-Guerin in the treatment of bladder cancer. Biomaterials. 2013;34:10258–66.
Guo HJ, Zhang DR, Li CY, Jia LJ, Liu GP, Hao LL, et al. Self-assembled nanoparticles based on galactosylated O-carboxymethyl chitosan-graft-stearic acid conjugates for delivery of doxorubicin. Int J Pharm. 2013;458:31–8.
Chicatun F, Pedraza CE, Muja N, Ghezzi CE, McKee MD, Nazhat SN. Effect of chitosan incorporation and scaffold geometry on chondrocyte function in dense collagen type I hydrogels. Tissue Eng A. 2013;19:2553–64.
Panyam J, Dali MA, Sahoo SK, Ma WX, Chakravarthi SS, Amidon GL, et al. Polymer degradation and in vitro release of a model protein from poly(D, L-lactide-co-glycolide) nano- and microparticles. J Control Release. 2003;92:173–87.
Suarez S, Grover GN, Braden RL, Christman KL, Amutairi A. Tunable protein release from acetalated dextran microparticles: a platform for delivery of protein therapeutics to the heart post-MI. Biomacromolecules. 2013;14:3927–35.
Couto LT, Donato JL, De Nucci G. Analysis of five streptokinase formulations using the euglobulin lysis test and the plasminogen activation assay. Braz J Med Biol Res. 2004;37:1889–94.
Hughes GA. Nanostructure-mediated drug delivery. Dis Mon. 2005;51:342–61.
Kathleen D, Vandervoort J, Van den Mooter G, Ludwig A. Evaluation of ciprofloxacin-loaded Eudragit((R)) RS100 or RL100/PLGA nanoparticles. Int J Pharm. 2006;314:72–82.
Wu JH, Siddiqui K, Diamond SL. Transport phenomena and clot dissolving therapy: an experimental investigation of diffusion-controlled and permeation-enhanced fibrinolysis. Thromb Haemost. 1994;72:105–12.
Staros JV, Wright RW, Swingle DM. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem. 1986;156:220–2.
Mustafin RI, Bodrov AV, Kemenova VA, Rombaut P, Van den Mooter G. Interpolymer interaction between countercharged types of Eudragit(A (R)) RL30D and FS30D in binary films as a method of drug release modification in oral delivery systems. Pharm Chem J. 2012;46:45–9.
Mahmoodi M, Khosroshahi ME, Atyabi F. Early experimental results of thrombolysis using controlled release of tissue plasminogen activator encapsulated by PLGA/CS nanoparticles delivered by pulse 532 nm laser. Dig J Nanomater Biostruct. 2011;6:889–905.
Chen JP, Yang PC, Ma YH, Wu T. Characterization of chitosan magnetic nanoparticles for in situ delivery of tissue plasminogen activator. Carbohydr Polym. 2011;84:364–72.
Jin HJ, Zhang H, Sun ML, Zhang BG, Zhang JW. Urokinase-coated chitosan nanoparticles for thrombolytic therapy: preparation and pharmacodynamics in vivo. J Thromb Thrombolysis. 2013;36:458–68.
Modaresi SMS, Mehr SE, Faramarzi MA, Gharehdaghi EE, Azarnia M, Modarressi MH, et al. Preparation and characterization of self-assembled chitosan nanoparticles for the sustained delivery of streptokinase: an in vivo study. Pharm Dev Technol. 2014;19:593–7.
Nguyen DA, Fogler HS. Facilitated diffusion in the dissolution of carboxylic polymers. AIChE J. 2005;51:415–25.
Chakravarthi SS, Robinson DH. Enhanced cellular association of paclitaxel delivered in chitosan-PLGA particles. Int J Pharm. 2011;409:111–20.
Blinc A, Francis CW. Transport processes in fibrinolysis and fibrinolytic therapy. Thromb Haemost. 1996;76:481–91.
Blinc A, Planinsic G, Keber D, Jarh O, Lahajnar G, Zidansek A, et al. Dependence of blood clot lysis on the mode of transport of urokinase into the clot—a magnetic resonance imaging study in vitro. Thromb Haemost. 1991;65:549–52.
Diamond SL, Anand S. Inner clot diffusion and permeation during fibrinolysis. Biophys J. 1993;65:2622–43.
Collet JP, Montalescot G, Lesty C, Weisel JW. A structural and dynamic investigation of the facilitating effect of glycoprotein IIb/IIIa inhibitors in dissolving platelet-rich clots. Circ Res. 2002;90:428–34.
Sakharov DV, Nagelkerke JF, Rijken DC. Rearrangements of the fibrin network and spatial distribution of fibrinolytic components during plasma clot lysis—study with confocal microscopy. J Biol Chem. 1996;271:2133–8.
Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35:2220–5.
Zehendner CM, Librizzi L, de Curtis M, Kuhlmann CRW, Luhmann HJ. Caspase-3 contributes to ZO-1 and Cl-5 tight-junction disruption in rapid anoxic neurovascular unit damage. PLoS ONE. 2011;6:11.
Ishii T, Fukuta T, Agato Y, Oyama D, Yasuda N, Shimizu K, et al. Nanoparticles accumulate in ischemic core and penumbra region even when cerebral perfusion is reduced. Biochem Biophys Res Commun. 2013;430:1201–5.
Cruz LJ, Stammes MA, Que I, van Beek ER, Knol-Blankevoort VT, Snoeks TJA, et al. Effect of PLGA NP size on efficiency to target traumatic brain injury. J Control Release. 2016;223:31–41.
Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–78.
Zolnik BS, Gonzalez-Fernandez A, Sadrieh N, Dobrovolskaia MA. Nanoparticles and the immune system. Endocrinology. 2010;151:458–65.
Ishak RAH, Awad GAS, Zaki NM, El-Shamy A, Mortada ND. A comparative study of chitosan shielding effect on nano-carriers hydrophilicity and biodistribution. Carbohydr Polym. 2013;94:669–76.
Ziemba B, Halets I, Shcharbin D, Appelhans D, Voit B, Pieszynski I, et al. Influence of fourth generation poly(propyleneimine) dendrimers on blood cells. J Biomed Mater Res A. 2012;100A:2870–80.
Jones CF, Campbell RA, Brooks AE, Assemi S, Tadjiki S, Thiagarajan G, et al. Cationic PAMAM dendrimers aggressively initiate blood clot formation. ACS Nano. 2012;6:9900–10.
Jones CF, Campbell RA, Franks Z, Gibson CC, Thiagarajan G, Vieira-de-Abreu A, et al. Cationic PAMAM dendrimers disrupt key platelet functions. Mol Pharm. 2012;9:1599–611.
Kohane DS, Anderson DG, Yu C, Langer R. pH-triggered release of macromolecules from spray-dried polymethacrylate microparticles. Pharm Res. 2003;20:1533–8.
Zago AC, Raudales JC, Attizzani G, Matte BS, Yamamoto GI, Balvedi JA, et al. Local delivery of sirolimus nanoparticles for the treatment of in-stent restenosis. Catheter Cardiovasc Interv. 2013;81:E124–9.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors gratefully acknowledge Dr. Preston Larson and Greg Strout from the Samuel Roberts Noble Electron Microscopy Laboratory at the University of Oklahoma for technical assistance with the SEM and TEM experiments. We are also grateful to Evonic Röhm for supplying Eudragit FS30D.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, H.X., O’Rear, E.A. An In Vitro Thrombolysis Study Using a Mixture of Fast-Acting and Slower Release Microspheres. Pharm Res 33, 1552–1563 (2016). https://doi.org/10.1007/s11095-016-1897-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-1897-1