Abstract
Purpose
The human pathogen Chlamydia trachomatis is worldwide the leading cause of bacterial sexually transmitted disease. Nasal or vaginal nucleic acid vaccination is a promising strategy for controlling genital Chlamydia trachomatis infections. Since naked nucleic acids are generally not efficiently taken up by cells, they are often complexed with carriers that facilitate their intracellular delivery.
Methods
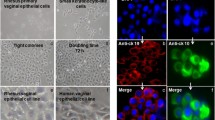
In the current study, we screened a variety of commonly used non-viral gene delivery carriers for their ability to transfect newborn pig tracheal cells. The effect of aerosolization on the physicochemical properties and transfection efficiency of the complexes was also evaluated in vitro. Subsequently, a pilot experiment was performed in which the selected complexes were aerosolized in the vaginal tract of pigs.
Results
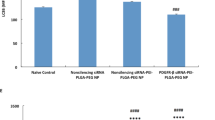
Both mRNA and pDNA containing lipofectamine and ADM70 complexes showed promise for protein expression in vitro, before and after aerosolization. In vivo, only lipofectamine/pDNA complexes resulted in high protein expression levels 24 h following aerosolization. This correlates to the unexpected observation that the presence of vaginal mucus increases the efficiency of lipofectamine/pDNA complexes 3-fold, while the efficiency of lipofectamine/mRNA complexes and ADM70/mRNA and ADM70/pDNA complexes decreased.
Conclusions
As aerosolization was an easy and effective method to deliver complexes to the vaginal tract of pigs, we believe this application technique has future potential for both vaginal and perhaps nasal vaccination using non-viral gene delivery vectors.






Similar content being viewed by others
Abbreviations
- C.:
-
Chlamydia
- DLS:
-
Dynamic light scattering
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DMPE:
-
1,2-Dimyristoyl-sn-glycero-3-phosphoethanolamine
- DMSO:
-
Dimethylsulfoxide
- DOPE:
-
Dioleoylphosphatidylethanolamine
- DOTAP:
-
1,2-dioleoyl-3-trimethylammonium-propane
- FCS:
-
Fetal calf serum
- GFP:
-
Green fluorescent protein
- GL67:
-
Genzyme lipid 67
- Luc:
-
Luciferase
- MTT:
-
3-(4,5-dimethylthiazole-2yl)-2,5-diphenyl tetrazolium bromide
- NPTr:
-
Newborn pig tracheal
- PEG:
-
Polyethylene glycol
- PEI:
-
Polyethyleneimine
- ROI:
-
Regions of interest
References
WHO. Global incidence and prevalence of selected curable sexually transmitted infections – 2008. Geneva: World Health Organization; 2012.
Peipert JF. Genital chlamydial infections. N Engl J Med. 2003;349:2424–30.
Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5(2):149–61.
Manavi K. A review on infection with Chlamydia trachomatis. Best Pract Res Clin Obstet Gynaecol. 2006;20(6):941–51.
Cunningham KA, Beagley KW. Male genital tract chlamydial infection: implications for pathology and infertility. Biol Reprod. 2008;79(2):180–9.
Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19(4):185–92.
Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis. 2010;201 Suppl 2:S134–55.
Plummer FA, Simonsen JN, Cameron DW, Ndinya-Achola JO, Kreiss JK, Gakinya MN, et al. Cofactors in male–female sexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1991;163(2):233–9.
Koskela P, Anttila T, Bjørge T, Brunsvig A, Dillner J, Hakama M, et al. Chlamydia trachomatis infection as a risk factor for invasive cervical cancer. Int J Cancer. 2000;85(1):35–9.
Samoff E, Koumans EH, Markowitz LE, Sternberg M, Sawyer MK, Swan D, et al. Association of Chlamydia trachomatis with persistence of high-risk types of human papillomavirus in a cohort of female adolescents. Am J Epidemiol. 2005;162(7):668–75.
Stagg AJ. Vaccines against Chlamydia: approaches and progress. Mol Med Today. 1998;4(4):166–73.
Hafner L, Beagley K, Timms P. Chlamydia trachomatis infection: host immune responses and potential vaccines. Mucosal Immunol. 2008;1(2):116–30.
Kanazawa T, Takashima Y, Shibata Y, Tsuchiya M, Tamura T, Okada H. Effective vaginal DNA delivery with high transfection efficiency is a good system for induction of higher local vaginal immune responses. J Pharm Pharmacol. 2009;61(11):1457–63.
Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11(4 Suppl):S45–53.
Wassén L, Schön K, Holmgren J, Jertborn M, Lycke N. Local intravaginal vaccination of the female genital tract. Scand J Immunol. 1996;44(4):408–14.
Johansson EL, Wassén L, Holmgren J, Jertborn M, Rudin A. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect Immun. 2001;69(12):7481–6.
Symens N, Soenen SJ, Rejman J, Braeckmans K, De Smedt SC, Remaut K. Intracellular partitioning of cell organelles and extraneous nanoparticles during mitosis. Adv Drug Deliv Rev. 2012;64(1):78–94.
Tavernier G, Andries O, Demeester J, Sanders NN, De Smedt SC, Rejman J. mRNA as gene therapeutic: how to control protein expression. J Control Release. 2011;150(3):238–47.
Remaut K, Sanders NN, De Geest BG, Braeckmans K, Demeester J, De Smedt SC. Nucleic acid delivery: where material sciences and bio-sciences meet. Mater Sci Eng R Rep. 2007;58(3–5):117–61.
Symens N, Méndez-Ardoy A, Díaz-Moscoso A, Sánchez-Fernández E, Remaut K, Demeester J, et al. Efficient transfection of hepatocytes mediated by mRNA complexed to galactosylated cyclodextrins. Bioconjug Chem. 2012;23(6):1276–89.
Ferrari M, Scalvini A, Losio MN, Corradi A, Soncini M, Bignotti E, et al. Establishment and characterization of two new pig cell lines for use in virological diagnostic laboratories. J Virol Methods. 2003;107(2):205–12.
Geall AJ, Mandl CW, Ulmer JB. RNA: the new revolution in nucleic acid vaccines. Semin Immunol. 2013;25(2):152–9.
Longbottom D, Livingstone M. Vaccination against chlamydial infections of man and animals. Vet J. 2006;171(2):263–75.
Devoldere J, Dewitte H, De Smedt SC, Remaut K. Evading innate immunity in nonviral mRNA delivery: don’t shoot the messenger. Drug Discov Today. 2015. doi:10.1016/j.drudis.2015.07.009.
Andries O, De Filette M, De Smedt SC, Demeester J, Van Poucke M, Peelman L, et al. Innate immune response and programmed cell death following carrier-mediated delivery of unmodified mRNA to respiratory cells. J Control Release. 2013;167(2):157–66.
Andries O, Cafferty SM, De Smedt SC, Weiss R, Sanders NN, Kitada T. N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J Control release. 2015;217:337–44.
Schautteet K, Stuyven E, Beeckman DS, Van Acker S, Carlon M, Chiers K, et al. Protection of pigs against Chlamydia trachomatis challenge by administration of a MOMP-based DNA vaccine in the vaginal mucosa. Vaccine. 2011;29(7):1399–407.
Schautteet K, De Clercq E, Jönsson Y, Lagae S, Chiers K, Cox E, et al. Protection of pigs against genital Chlamydia trachomatis challenge by parenteral or mucosal DNA immunization. Vaccine. 2012;30(18):2869–81.
Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol. 2010;10(10):699–711.
Bettinger T, Carlisle RC, Read ML, Ogris M, Seymour LW. Peptide-mediated RNA delivery: a novel approach for enhanced transfection of primary and post-mitotic cells. Nucleic Acids Res. 2001;29(18):3882–91.
Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16(11):1833–40.
Capecchi MR. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980;22(2 Pt 2):479–88.
Mirzayans R, Aubin RA, Paterson MC. Differential expression and stability of foreign genes introduced into human fibroblasts by nuclear versus cytoplasmic microinjection. Mutat Res. 1992;281(2):115–22.
Thorburn AM, Alberts AS. Efficient expression of miniprep plasmid DNA after needle micro-injection into somatic cells. Biotechniques. 1993;14(3):356–8.
Zabner J, Fasbender AJ, Moninger T, Poellinger KA, Welsh MJ. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem. 1995;270(32):18997–9007.
Martens TF, Remaut K, Demeester J, De Smedt SC, Braeckmans K. Intracellular delivery of nanomaterials: How to catch endosomal escape in the act. Nano Today. 2014;9(3):344–64.
Zu R, Zuhorn IS, Hoekstra D. How cationic lipids transfer nucleic acids into cells and across cellular membranes: recent advances. J Control Release. 2013;166(1):46–56.
Zu R, Hoekstra D, Zuhorn IS. Mechanism of polyplex- and lipoplex-mediated delivery of nucleic acids: real-time visualization of transient membrane destabilization without endosomal lysis. ACS Nano. 2013;7(5):3767–77.
Andries O, De Filette M, Rejman J, De Smedt SC, Demeester J, Van Poucke M, et al. Comparison of the gene transfer efficiency of mRNA/GL67 and pDNA/GL67 complexes in respiratory cells. Mol Pharm. 2012;9(8):2136–45.
Ponsaerts P, Van Tendeloo VFI, Berneman ZN. Cancer immunotherapy using RNA-loaded dendritic cells. Clin Exp Immunol. 2003;134(3):378–84.
Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004;199:251–63.
Ulmer JB, Mason PW, Geall A, Mandl CW. RNA-based vaccines. Vaccine. 2012;30(30):4414–8.
ACKNOWLEDGMENTS AND DISCLOSURES
Oliwia Andries is a doctoral fellow of the Research Foundation Flanders. Their financial support is acknowledged with gratitude. E. De Clercq has a PhD fellowship from the Special Research Fund of Ghent University. We also acknowledge funding support from the Research Foundation Flanders (FWO grant n° G.0621.10) and the Special Research Fund (BOF) of Ghent University. Dr. Bakr Ahmed is acknowledged for the collection of pig’s cervical mucus. J. M. García Fernández and J. M. Benito acknowledge financial support from the Junta de Andalucía (contract number FQM2012-1467), and the European Regional Development Funds (FEDER).
Compliance with Ethical Standards
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Ghent University).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Niek Sanders and Daisy Vanrompay contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Suppl Fig 1
Cell viability. NPTr cells were treated with different mRNA/carrier complexes as depicted in the x-axis. Blanc represents non-transfected cells and DMSO served as positive control. * Significantly different from non-treated cells (blanc) (P ≤ 0.05). (JPG 1.12 mb)
Rights and permissions
About this article
Cite this article
Remaut, K., De Clercq, E., Andries, O. et al. Aerosolized Non-viral Nucleic Acid Delivery in the Vaginal Tract of Pigs. Pharm Res 33, 384–394 (2016). https://doi.org/10.1007/s11095-015-1796-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1796-x