Abstract
Purpose
Nanostructured Lipid Carriers (NLCs) loading oxaprozin were developed to address an effective drug packaging and targeted delivery, improving the drug pharmacokinetics and pharmacodynamics properties and avoiding the local gastric side-effects. Macrophages actively phagocyte particles with sizes larger than 200 nm and, when activated, over-express folate beta receptors - features that in the case of this work constitute the basis for passive and active targeting strategies.
Methods
Two formulations containing oxaprozin were developed: NLCs with and without folate functionalization. In order to target the macrophages folate receptors, a DSPE-PEG2000-FA conjugate was synthesized and added to the NLCs.
Results
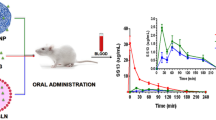
These formulations presented a relatively low polydispersity index (approximately 0.2) with mean diameters greater than 200 nm and zeta potential inferior to −40 mV. The encapsulation efficiency of the particles was superior to 95% and the loading capacity was of 9%, approximately. The formulations retained the oxaprozin release in simulated gastric fluid (only around 10%) promoting its release on simulated intestinal fluid. MTT and LDH assays revealed that the formulations only presented cytotoxicity in Caco-2 cells for oxaprozin concentrations superior to 100 μM. Permeability studies in Caco-2 cells shown that oxaprozin encapsulation did not interfered with oxaprozin permeability (around 0.8 × 10−5 cm/s in simulated intestinal fluid and about 1.45 × 10−5 cm/s in PBS). Moreover, in RAW 264.7 cells NLCs functionalization promoted an increased uptake over time mainly mediated by a caveolae uptake mechanism.
Conclusions
The developed nanoparticles enclose a great potential for oxaprozin oral administration with significant less gastric side-effects.










Similar content being viewed by others
Abbreviations
- DSPE-PEG2000-FA:
-
Disteroylphosphatidylethanolamine-poly(ethylene glycol)2000-folic acid
- EE:
-
Encapsulation efficiency
- FA-NLCs + Oxa:
-
DSPE-PEG2000-FA functionalized oxaprozin loaded nanostructured lipid carriers NLCs
- FA-NLCs:
-
DSPE-PEG2000-FA functionalized placebo nanostructured lipid carriers
- FR:
-
Folate receptor
- GI:
-
Gastrointestinal
- LC:
-
Loading capacity
- LNs:
-
Lipid nanoparticles
- NLCs:
-
Nanostructured lipid carriers
- NLCs Placebo:
-
Non-functionalized placebo nanostructured lipid carriers
- NLCs + Oxa:
-
Non-functionalized oxaprozin loaded nanostructured lipid carriers
- NSAIDs:
-
Non-steroidal Anti-inflammatory drugs
- Oxa:
-
Oxaprozin
- SLN:
-
Solid lipid nanoparticles
- TEER:
-
Transepithelial electrical resistance
References
Bruno A, Tacconelli S, Patrignani P. Variability in the Response to Non-Steroidal Anti-Inflammatory Drugs: Mechanisms and Perspectives. Basic Clin Pharmacol Toxicol. 2014;114(1):56–63.
Moilanen E. Two Faces of Inflammation: An Immunopharmacological View. Basic Clin Pharmacol Toxicol. 2014;114(1):2–6.
Rao PPN, Kabir SN, Mohamed T. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): Progress in Small Molecule Drug Development. Pharmaceuticals. 2010;3(5):1530–49.
Qandil A. Prodrugs of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), More Than Meets the Eye: A Critical Review. Int J Mol Sci. 2012;13(12):17244–74.
Kean WF, Buchanan WW. The use of NSAIDs in rheumatic disorders 2005: a global perspective. Inflammopharmacology. 2005;13(4):343–70.
Knights KM, Mangoni AA, Miners JO. Defining the COX inhibitor selectivity of NSAIDs: implications for understanding toxicity. Expert Rev Clin Pharmacol. 2010;3(6):769–76.
Vane JR, Botting RM. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104(3A):2S–8.
Shaji J, Lal M. Nanocarriers for targeting in inflammation. Asian J Pharm Clin Res. 2013;6:3–12.
Hua S. Targeting sites of inflammation: intercellular adhesion molecule-1 as a target for novel inflammatory therapies. Front Pharmacol. 2013;4:127.
Crielaard BJ, Lammers T, Schiffelers RM, Storm G. Drug targeting systems for inflammatory disease: one for all, all for one. J Control Release: Off J Control Release Soc. 2012;161(2):225–34.
Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–27.
Ulbrich W, Lamprecht A. Targeted drug-delivery approaches by nanoparticulate carriers in the therapy of inflammatory diseases. J R Soc Interface R Soc. 2010;7 Suppl 1:S55–66.
Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56(8):1067–84.
Leamon CP, Low PS. Delivery of macromolecules into living cells: a method that exploits folate receptor endocytosis. Proc Natl Acad Sci. 1991;88(13):5572–6.
Wissing SA, Kayser O, Müller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56(9):1257–72.
Das S, Chaudhury A. Recent Advances in Lipid Nanoparticle Formulations with Solid Matrix for Oral Drug Delivery. AAPS PharmSciTech. 2011;12(1):62–76.
Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366(1–2):170–84.
Khan AA, Mudassir J, Mohtar N, Darwis Y. Advanced drug delivery to the lymphatic system: lipid-based nanoformulations. Int J Nanomedicine. 2013;8:2733–44.
Severino P, Andreani T, Macedo AS, Fangueiro JF, Santana MH, Silva AM, et al. Current State-of-Art and New Trends on Lipid Nanoparticles (SLN and NLC) for Oral Drug Delivery. J Drug Deliv. 2012;2012:750891.
Weber S, Zimmer A, Pardeike J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: A review of the state of the art. Eur J Pharm Biopharm. 2014;86(1):7–22.
Mehnert W, Mäder K. Solid lipid nanoparticles: Production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2–3):165–96.
Gamboa JM, Leong KW. In vitro and in vivo models for the study of oral delivery of nanoparticles. Adv Drug Deliv Rev. 2013;65(6):800–10.
Van de Graaff KM. Anatomy and physiology of the gastrointestinal tract. Pediatr Infect Dis. 1986;5(1 Suppl):S11–6.
Woodley JF. Enzymatic barriers for GI peptide and protein delivery. Crit Rev Ther Drug Carrier Syst. 1994;11(2–3):61–95.
Rainsford KD, Omar H, Ashraf A, Hewson AT, Bunning RAD, Rishiraj R, et al. Recent pharmacodynamic and pharmacokinetic findings on oxaprozin. Inflammopharmacology. 2002;10(3):185–239.
Bozic BD, Rogan JR, Poleti DD, Trisovic NP, Uscumlic GS. Synthesis, characterization and antiproliferative activity of transition metal complexes with 3-(4,5-diphenyl-1,3-oxazol-2-yl)propanoic acid (oxaprozin). Chem Pharm Bull. 2012;60(7):865–9.
Chan P, Kurisawa M, Chung JE, Yang Y-Y. Synthesis and characterization of chitosan-g-poly(ethylene glycol)-folate as a non-viral carrier for tumor-targeted gene delivery. Biomaterials. 2007;28(3):540–9.
van Steenis JH, van Maarseveen EM, Verbaan FJ, Verrijk R, Crommelin DJA, Storm G, et al. Preparation and characterization of folate-targeted pEG-coated pDMAEMA-based polyplexes. J Control Release. 2003;87(1–3):167–76.
Woitiski CB, Sarmento B, Carvalho RA, Neufeld RJ, Veiga F. Facilitated nanoscale delivery of insulin across intestinal membrane models. Int J Pharm. 2011;412(1–2):123–31.
Benfer M, Kissel T. Cellular uptake mechanism and knockdown activity of siRNA-loaded biodegradable DEAPA-PVA-g-PLGA nanoparticles. Eur J Pharm Biopharm. 2012;80(2):247–56.
Serda RE, Gu J, Bhavane RC, Liu X, Chiappini C, Decuzzi P, et al. The association of silicon microparticles with endothelial cells in drug delivery to the vasculature. Biomaterials. 2009;30(13):2440–8.
Zhang Z, Yao J. Preparation of Irinotecan-Loaded Folate-Targeted Liposome for Tumor Targeting Delivery and Its Antitumor Activity. AAPS PharmSciTech. 2012;13(3):802–10.
Lee Y-k. Preparation and characterization of folic acid linked poly(L-glutamate) nanoparticles for cancer targeting. Macromol Res. 2006;14(3):387–93.
Wang B, Gao Y, Li H-W, Hu Z-P, Wu Y. The switch-on luminescence sensing of histidine-rich proteins in solution: a further application of a Cu2+ ligand. Org Biomol Chem. 2011;9(11):4032–4.
Paranjpe PV, Chen Y, Kholodovych V, Welsh W, Stein S, Sinko PJ. Tumor-targeted bioconjugate based delivery of camptothecin: design, synthesis and in vitro evaluation. J Control Release. 2004;100(2):275–92.
Bonechi C, Donati A, Lampariello R, Martini S, Picchi MP, Ricci M, et al. Solution structure of folic acid: Molecular mechanics and NMR investigation. Spectrochim Acta A Mol Biomol Spectrosc. 2004;60(7):1411–9.
Gabizon A, Horowitz AT, Goren D, Tzemach D, Mandelbaum-Shavit F, Qazen MM, et al. Targeting Folate Receptor with Folate Linked to Extremities of Poly(ethylene glycol)-Grafted Liposomes: In Vitro Studies. Bioconjug Chem. 1999;10(2):289–98.
Neves AR, Lucio M, Martins S, Lima JL, Reis S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int J Nanomedicine. 2013;8:177–87.
Honary S, Zahir F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems - A Review (Part 2). Trop J Pharm Res. 2013;12(2):265–73.
Maestrelli F, Cirri M, Mennini N, Zerrouk N, Mura P. Improvement of oxaprozin solubility and permeability by the combined use of cyclodextrin, chitosan, and bile components. Eur J Pharm Biopharm. 2011;78(3):385–93.
Gan L-SL, Thakker DR. Applications of the Caco-2 model in the design and development of orally active drugs: elucidation of biochemical and physical barriers posed by the intestinal epithelium. Adv Drug Deliv Rev. 1997;23(1–3):77–98.
Schwebel HJ, van Hoogevest P, Leigh ML, Kuentz M. The apparent solubilizing capacity of simulated intestinal fluids for poorly water-soluble drugs. Pharm Dev Technol. 2011;16(3):278–86.
Frank KJ, Westedt U, Rosenblatt KM, Holig P, Rosenberg J, Magerlein M, et al. Impact of FaSSIF on the solubility and dissolution-/permeation rate of a poorly water-soluble compound. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2012;47(1):16–20.
Yazdanian M, Briggs K, Jankovsky C, Hawi A. The “high solubility” definition of the current FDA Guidance on Biopharmaceutical Classification System may be too strict for acidic drugs. Pharm Res. 2004;21(2):293–9.
Ehrhardt C, Kim KJ. Drug Absorption Studies: In Situ, In Vitro and In Silico Models. New York: Springer; 2007.
Lin Y-H, Mi F-L, Chen C-T, Chang W-C, Peng S-F, Liang H-F, et al. Preparation and Characterization of Nanoparticles Shelled with Chitosan for Oral Insulin Delivery. Biomacromolecules. 2007;8(1):146–52.
Kandarova H. Evaluation and Validation of Reconstructed Human Skin Models as Alternatives to Animal Tests in Regulatory Toxicology. In: Biowissenschaften; Biologie. Freien Universität Berlin; 2006.
Thompson C, Cheng WP, Gadad P, Skene K, Smith M, Smith G, et al. Uptake and transport of novel amphiphilic polyelectrolyte-insulin nanocomplexes by Caco-2 cells--towards oral insulin. Pharm Res. 2011;28(4):886–96.
Yuan H, Chen C-Y, Chai G-h DY-Z, Hu F-Q. Improved Transport and Absorption through Gastrointestinal Tract by PEGylated Solid Lipid Nanoparticles. Mol Pharm. 2013;10(5):1865–73.
Huth US, Schubert R, Peschka-Süss R. Investigating the uptake and intracellular fate of pH-sensitive liposomes by flow cytometry and spectral bio-imaging. J Control Release. 2006;110(3):490–504.
Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58(1):32–45.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was funded by FEDER funds through the Operational Programme for Competitiveness Factors - COMPETE and by National Funds through FCT - Foundation for Science and Technology under the Pest-C/EQB/LA0006/2013 and FCOMP-01-0124-FEDER-3728. The work also received financial support from the European Union (FEDER funds) under the framework of QREN through Project NORTE-07-0162-FEDER-000088. To all financing sources the authors are greatly indebted. JLA, ARN and CN also acknowledge the FCT for financial support through the Research grant PD/BI/105914/2014, PhD grant SFRH/BD/73379/2010 and Post-Doc Grant SFRH/BPD/81963/2011.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lopes-de-Araújo, J., Neves, A.R., Gouveia, V.M. et al. Oxaprozin-Loaded Lipid Nanoparticles towards Overcoming NSAIDs Side-Effects. Pharm Res 33, 301–314 (2016). https://doi.org/10.1007/s11095-015-1788-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1788-x