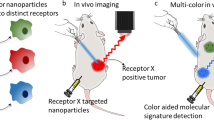
Abstract
Purpose
The objective of this study was to formulate nanoparticles of D-luciferin (Nano-Luc), DiR (Nano-DiR) and dual functional nanoparticles with DiR and luciferin (Nano-LucDiR) for in-vivo imaging as well as tracking of the nanoparticles in tumors.
Methods
Nano-Luc and Nano-LucDiR were prepared using different lipids, and subsequently characterized for loading and entrapment efficiency, physical properties, release profile, toxicity and stability. We utilized Response Surface Methodology (RSM) to optimize the nanoparticles using design of experiment (DOE Vr.8.0). Nano-Luc was evaluated against free luciferin to establish its pharmacokinetic parameters in mice. In-vivo imaging of tumors and tracking of nanoparticles was carried out with an IVIS® Spectrum-CT (Caliper) using xenograft, orthotopic and metastatic tumor models in BALB/c nude mice with different cell lines and different routes of nanoparticle administration (subcutaneous, intraperitoneal and intravenous).
Results
Particle size of both Nano-Luc and Nano-LucDiR were found to be <200 nm. Nano-Luc formulation showed a slow and controlled release upto 72 h (90%) in vitro. The optimized Nano-Luc had loading efficiency of 5.0 mg/ml with 99% encapsulation efficiency. Nano-Luc and Nano-LucDiR formulations had good shelf stability. Nano-Luc and Nano-LucDiR enhanced plasma half-life of luciferin compared to free luciferin thus providing longer circulation of luciferin in plasma enabling imaging of tumors for more than 24 h. Nano-LucDiR allowed simultaneous bioluminescent and fluorescent imaging to be conducted, with three-dimensional reconstruct of tumors without losing either signal during the acquisition time.
Conclusion
Nano-Luc and Nano-LucDiR allowed prolonged reproducible in-vivo imaging of tumors, especially during multimodality 3D imaging.





Similar content being viewed by others
Abbreviations
- CCD:
-
Central Composite Design
- CT:
-
Computed Tomography
- DOE:
-
Design Of Experiment
- DSC:
-
Differential Scanning Calorimetry
- FOV:
-
Field Of View
- IP:
-
Intraperitoneal
- IV:
-
Intravenous
- Nano-DiR:
-
Nanoparticles of DiR
- Nano-Luc:
-
Nanoparticles of D-Luciferin
- Nano-LucDiR:
-
Nanoparticles with DiR and Luciferin
- NCs:
-
Nano lipid carriers
- QbB:
-
Quality by Design
- ROI:
-
Region of Interest
- RSM:
-
Response Surface Methodology
- SD:
-
Standard Deviations
- SQ/SC:
-
Subcutaneous
- TPGS:
-
α-Tocopherol Polyethylene Glycol Succinate
- Xenolight DiR:
-
DiIC18(7) or 1,1′-dioctadecyltetramethyl indotricarbocyanine iodide
REFERENCES
Di Carli MF, Dorbala S, Meserve J, El Fakhri G, Sitek A, Moore SC. Clinical myocardial perfusion PET/CT. J Nucl Med. 2007;48(5):783–93.
Mosconi L, Tsui WH, Herholz K, Pupi A, Drzezga A, Lucignani G, et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med. 2008;49(3):390–8.
Zhao B, Schwartz LH, Larson SM. Imaging surrogates of tumor response to therapy: anatomic and functional biomarkers. J Nucl Med. 2009;50(2):239–49.
Hillner BE, Liu D, Coleman RE, Shields AF, Gareen IF, Hanna L, et al. The National Oncologic PET Registry (NOPR): design and analysis plan. J Nucl Med. 2007;48(11):1901–8.
Hargreaves RJ. The role of molecular imaging in drug discovery and development. Clin Pharmacol Ther. 2008;83(2):349–53.
Niu G, Chen X. Has molecular and cellular imaging enhanced drug discovery and drug development? Drugs R D. 2008;9(6):351–68.
Cho H, Ackerstaff E, Carlin S, Lupu ME, Wang Y, Rizwan A, et al. Noninvasive multimodality imaging of the tumor microenvironment: registered dynamic magnetic resonance imaging and positron emission tomography studies of a preclinical tumor model of tumor hypoxia. Neoplasia. 2009;11(3):247–59. 2p following 59.
Contag CH, Ross BD. It’s not just about anatomy: in vivo bioluminescence imaging as an eyepiece into biology. J Magn Reson Imaging. 2002;16(4):378–87.
Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49 Suppl 2:113S–28.
Lim E, Modi K, Christensen A, Meganck J, Oldfield S, Zhang N. Monitoring tumor metastases and osteolytic lesions with bioluminescence and micro CT imaging. J Vis Exp. 2011(50):2775.
Gross S, Piwnica-Worms D. Spying on cancer: molecular imaging in vivo with genetically encoded reporters. Cancer Cell. 2005;7(1):5–15.
Corn DJ, Kim Y, Krebs MD, Mounts T, Molter J, Gerson S, et al. Imaging Early Stage Osteogenic Differentiation of Mesenchymal Stem Cells. J Orthop Res. 2013;31(6):871–9.
Chan CT, Paulmurugan R, Reeves RE, Solow-Cordero D, Gambhir SS. Molecular imaging of phosphorylation events for drug development. Mol Imaging Biol. 2009;11(3):144–58.
Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nat Rev Drug Discov. 2008;7(7):591–607.
Hayashi D, Tkacz JN, Hammond S, Devenney-Cakir BC, Zaim S, Bouzegaou N, et al. Gastroenteropancreatic neuroendocrine tumors: multimodality imaging features with pathological correlation. Jpn J Radiol. 2011;29(2):85–91.
Zinn KR, Chaudhuri TR, Szafran AA, O’Quinn D, Weaver C, Dugger K, et al. Noninvasive bioluminescence imaging in small animals. ILAR J. 2008;49(1):103–15.
Sadikot RT, Blackwell TS. Bioluminescence imaging. Proc Am Thorac Soc. 2005;2(6):537–40. 11–2.
Badr CE, Tannous BA. Bioluminescence imaging: progress and applications. Trends Biotechnol. 2011;29(12):624–33.
Zhang N, Lyons S, Lim E, Lassota P. A spontaneous acinar cell carcinoma model for monitoring progression of pancreatic lesions and response to treatment through noninvasive bioluminescence imaging. Clin Cancer Res. 2009;15(15):4915–24.
Zhang W, Feng JQ, Harris SE, Contag PR, Stevenson DK, Contag CH. Rapid in vivo functional analysis of transgenes in mice using whole body imaging of luciferase expression. Transgenic Res. 2001;10(5):423–34.
Lim E, Modi KD, Kim J. In vivo bioluminescent imaging of mammary tumors using IVIS spectrum. J Vis Exp. 2009(26):1210.
Bhaumik S, Gambhir SS. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc Natl Acad Sci U S A. 2002;99(1):377–82.
Frackman S, Anhalt M, Nealson KH. Cloning, organization, and expression of the bioluminescence genes of Xenorhabdus luminescens. J Bacteriol. 1990;172(10):5767–73.
Siragusa GR, Nawotka K, Spilman SD, Contag PR, Contag CH. Real-time monitoring of Escherichia coli O157:H7 adherence to beef carcass surface tissues with a bioluminescent reporter. Appl Environ Microbiol. 1999;65(4):1738–45.
Zhao H, Doyle TC, Coquoz O, Kalish F, Rice BW, Contag CH. Emission spectra of bioluminescent reporters and interaction with mammalian tissue determine the sensitivity of detection in vivo. J Biomed Opt. 2005;10(4):41210.
Gross S, Abraham U, Prior JL, Herzog ED, Piwnica-Worms D. Continuous delivery of D-luciferin by implanted micro-osmotic pumps enables true real-time bioluminescence imaging of luciferase activity in vivo. Mol Imaging. 2007;6(2):121–30.
Hiler DJ, Greenwald ML, Geusz ME. Imaging gene expression in live transgenic mice after providing luciferin in drinking water. Photochem Photobiol Sci. 2006;5(11):1082–5.
Berger F, Paulmurugan R, Bhaumik S, Gambhir SS. Uptake kinetics and biodistribution of 14C-D-luciferin–a radiolabeled substrate for the firefly luciferase catalyzed bioluminescence reaction: impact on bioluminescence based reporter gene imaging. Eur J Nucl Med Mol Imaging. 2008;35(12):2275–85.
Gross S, Piwnica-Worms D. Real-time imaging of ligand-induced IKK activation in intact cells and in living mice. Nat Methods. 2005;2(8):607–14.
Kheirolomoom A, Kruse DE, Qin S, Watson KE, Lai CY, Young LJ, et al. Enhanced in vivo bioluminescence imaging using liposomal luciferin delivery system. J Control Release. 2010;141(2):128–36.
Souto EB, Wissing SA, Barbosa CM, Muller RH. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm. 2004;278(1):71–7.
Kakkar V, Muppu SK, Chopra K, Kaur IP. Curcumin loaded solid lipid nanoparticles: an efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur J Pharm Biopharm. 2013;85(3 Pt A):339–45.
Muller RH, Radtke M, Wissing SA. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 2002;242(1–2):121–8.
Zhang GJ, Chen TB, Davide J, Tao W, Vanko A, Connolly B, et al. Visualization of Mitotic Arrest of Cell Cycle with Bioluminescence Imaging in Living Animals. Mol Imaging Biol. 2013 Feb 26.
Pardeike J, Hommoss A, Muller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366(1–2):170–84.
Singh B, Bhatowa R, Tripathi CB, Kapil R. Developing micro-/nanoparticulate drug delivery systems using “design of experiments”. Int J Pharm Investig. 2011;1(2):75–87.
Xiang QY, Wang MT, Chen F, Gong T, Jian YL, Zhang ZR, et al. Lung-targeting delivery of dexamethasone acetate loaded solid lipid nanoparticles. Arch Pharm Res. 2007;30(4):519–25.
Singh A, Ahmad I, Akhter S, Jain GK, Iqbal Z, Talegaonkar S, et al. Nanocarrier based formulation of Thymoquinone improves oral delivery: Stability assessment, in vitro and in vivo studies. Colloids Surf B: Biointerfaces. 2013;102:822–32.
Hao J, Wang F, Wang X, Zhang D, Bi Y, Gao Y, et al. Development and optimization of baicalin-loaded solid lipid nanoparticles prepared by coacervation method using central composite design. Eur J Pharm Sci. 2012;47(2):497–505.
Patlolla RR, Chougule M, Patel AR, Jackson T, Tata PN, Singh M. Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers. J Control Release. 2010;144(2):233–41.
Schwarz C, Mehnert W. Freeze-drying of drug-free and drug-loaded solid lipid nanoparticles (SLN). Int J Pharm. 1997;157(2):171–9.
Venkateswarlu V, Manjunath K. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J Control Release. 2004;95(3):627–38.
Puglia C, Blasi P, Rizza L, Schoubben A, Bonina F, Rossi C, et al. Lipid nanoparticles for prolonged topical delivery: an in vitro and in vivo investigation. Int J Pharm. 2008;357(1–2):295–304.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was financially supported by National Institute of Health - MBRS-SC1 Program (Grant # SC1 GM092779-01). The authors report no financial interest that might pose a potential, perceived, or real conflict of interest. U.S. Patent filed “Modified Nanodelivery System and Method for Enhanced In vivo Medical and Preclinical Imaging.” (U.S. 13/851610).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Affect of Lipid and Oil type on loading efficiency (A), entrapment efficiency (B), 24 h release rate (C) and mean particle size (D) in the RSM. (GIF 291 kb)
Figure S2
Response surface plots (A) and countor plots (B) showing the effect of different concentration of lipid and oil on release rate, loading efficiency and entrapment efficiency of Nano-Luc. (GIF 529 kb)
Figure S3
Differential Scanning Calorimetry of Nano-Luc formulations. A) Luciferin, B) Geleol, C) Nano-Luc, D) Precirol, E) Nano-LucDiR and F) Miglyol. (GIF 275 kb)
Figure S4
A) A graph of Log (% luciferin recovery) vs time for the effect of temperature (30°C, 40°C and 50°C) on Nano-Luc stability. B) In-vitro Comparison of Nano-luciferin and luciferin at 150 μg/mL with background subtraction (Plot of total flux Vs cell number). (n = 3) (GIF 228 kb)
Figure S5
Nano-luc Tumor Imaging in mice with A) subcutaneous 4 T1-luc2 tumors and B) orthotopic 4 T1-luc2 tumors using 150 mg/kg luciferin equivalent Nano-Luc by IP. (GIF 232 kb)
ESM 1
(DOCX 53 kb)
ESM 2
(DOCX 183 kb)
ESM 3
(DOCX 86 kb)
ESM 4
(DOCX 53 kb)
ESM 5
(AVI 6358 kb)
Rights and permissions
About this article
Cite this article
Patel, A.R., Lim, E., Francis, K.P. et al. Opening Up the Optical Imaging Window Using Nano-Luciferin. Pharm Res 31, 3073–3084 (2014). https://doi.org/10.1007/s11095-014-1400-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1400-9