ABSTRACT
Purpose
When information is sparse, individual parameters derived from a non-linear mixed effects model analysis can shrink to the mean. The objective of this work was to predict individual parameter shrinkage from the Bayesian information matrix (M BF ). We 1) Propose and evaluate an approximation of M BF by First-Order linearization (FO), 2) Explore by simulations the relationship between shrinkage and precision of estimates and 3) Evaluate prediction of shrinkage and individual parameter precision.
Methods
We approximated M BF using FO. From the shrinkage formula in linear mixed effects models, we derived the predicted shrinkage from M BF . Shrinkage values were generated for parameters of two pharmacokinetic models by varying the structure and the magnitude of the random effect and residual error models as well as the design. We then evaluated the approximation of M BF FO and compared it to Monte-Carlo (MC) simulations. We finally compared expected and observed shrinkage as well as the predicted and estimated Standard Errors (SE) of individual parameters.
Results
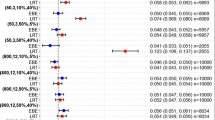
M BF FO was similar to M BF MC. Predicted and observed shrinkages were close . Predicted and estimated SE were similar.
Conclusions
M BF FO enables prediction of shrinkage and SE of individual parameters. It can be used for design optimization.






Similar content being viewed by others
Abbreviations
- CL:
-
Clearance
- CV:
-
Coefficient of Variation
- FO:
-
First-Order linearization
- IU:
-
International Units
- Km :
-
Michaelis-Menten constant
- MAP:
-
Maximum A Posteriori
- M BF :
-
Bayesian Fisher Information Matrix
- MC:
-
Monte-Carlo
- M F :
-
Fisher Information Matrix
- M IBF :
-
Individual Bayesian Fisher Information Matrix
- M IF :
-
Individual Fisher Information Matrix
- ML:
-
Maximum Likelihood
- MONOLIX:
-
MOdèles NOn LInéaires à effets miXtes
- NLMEM:
-
Non-Linear Mixed Effects Models
- PK:
-
Pharmacokinetics
- Q:
-
Intercompartmental clearance
- RSE:
-
Relative Standard Errors
- SE:
-
Standard Errors
- Sh:
-
Shrinkage
- V:
-
Volume of distribution
- V1-V2 :
-
Volume of distribution of the central and peripheral compartment
- Vm :
-
Maximum elimination rate
REFERENCES
Pillai GC, Mentré F, Steimer J-L. Non-linear mixed effects modeling - from methodology and software development to driving implementation in drug development science. J Pharmacokinet Pharmacodyn. 2005;32(2):161–83.
Beal S, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM User’s guides. (1989–2009), Icon development Solutions, Ellicott City, USA 2009.
Al-Banna MK, Kelman AW. Experimental design and efficient parameter estimation in population pharmacokinetics. J Pharmacokinet Biopharm. 1990;18(4):347–60.
Holford NH, Kimko HC, Monteleone JP, Peck CC. Simulation of clinical trials. Annu Rev Pharmacol Toxicol. 2000;40:209–34.
Atkinson AC, Donev AN. Optimum experimental designs. Oxford: Oxford University Press; 1992.
Fisher RA. The logic of inductive inference. J R Stat Soc. 1935;98(1):39–54.
Mentré F, Mallet A, Baccar D. Optimal design in random-effects regression models. Biometrika. 1997;84(2):429–42.
Retout S, Mentré F, Bruno R. Fisher information matrix for non-linear mixed-effects models: evaluation and application for optimal design of enoxaparin population pharmacokinetics. Stat Med. 2002;21(18):2623–39.
Bazzoli C, Retout S, Mentré F. Design evaluation and optimisation in multiple response non-linear mixed effect models: PFIM 3.0. Comput Methods Prog Biomed. 2010;98(1):55–65.
Nyberg J, Ueckert S, Strömberg EA, Hennig S, Karlsson MO, Hooker AC. PopED: an extended, parallelized, nonlinear mixed effects models optimal design tool. Comput Methods Prog Biomed. 2012;108(2):789–805.
Gueorguieva I, Ogungbenro K, Graham G, Glatt S, Aarons L. A program for individual and population optimal design for univariate and multivariate response pharmacokinetic-pharmacodynamic models. Comput Methods Prog Biomed. 2007;86(1):51–61.
Foo LK, Duffull S. Adaptive optimal design for bridging studies with an application to population pharmacokinetic studies. Pharm Res. 2012;29(6):1530–43.
Foo LK, Duffull S. Methods of robust design of non-linear models with an application to pharmacokinetics. J Biopharm Stat. 2010;20(4):886–902.
Retout S, Mentré F. Optimization of individual and population designs using Splus. J Pharmacokinet Pharmacodyn. 2003;30(6):417–43.
Merlé Y, Mentré F. Bayesian design criteria: computation, comparison, and application to a pharmacokinetic and a pharmacodynamic model. J Pharmacokinet Biopharm. 1995;23(1):101–25.
Sheiner LB, Beal S, Rosenberg B, Marathe VV. Forecasting individual pharmacokinetics. Clin Pharmacol Ther. 1979;26(3):294–305.
Sheiner LB, Beal SL. Bayesian individualization of pharmacokinetics: simple implementation and comparison with non-Bayesian methods. J Pharm Sci. 1982;71(12):1344–8.
Fedorov F. Mixed models: design of experiments. Presented at Design and analysis of experiments, Cambridge, UK. 11th August 2011 (http://www.newton.ac.uk/programmes/DAE/seminars/081109301.html).
Savic RM, Karlsson MO. Importance of shrinkage in empirical bayes estimates for diagnostics: problems and solutions. AAPS J. 2009;11(3):558–69.
Mentré F, Burtin P, Merlé Y, Van Bree J, Mallet A, Steimer J-L. Sparse-sampling optimal designs in pharmacokinetics and toxicokinetics. Drug Inf J. 1995;29(3):997–1019.
Frey N, Grange S, Woodworth T. Population pharmacokinetic analysis of tocilizumab in subjects with rheumatoid arthritis. J Clin Pharmacol. 2010;50(7):754–66.
Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer; 2000.
Pilz J. Bayesian estimation and experimental design in linear regression models. Leipzig: Teubner-Texte; 1983.
Boeckmann AJ, Sheiner LB, Beal SL. NONMEM users guide – Part V introductory guide. Ellicott City: Icon development Solutions; 2011.
McGree JM, Eccleston JA, Duffull SB. Compound optimal design criteria for nonlinear models. J Biopharm Stat. 2008;18(4):646–61.
Jones B, Wang J. Constructing optimal designs for fitting pharmacokinetic models. Stat Comput. 1999;9(3):209–18.
Jones B, Wang J, Jarvis P, Byrom W. Design of cross-over trials for pharmacokinetic studies. J Stat Plan Infer. 1999;78(1–2):307–16.
ACKNOWLEDGMENTS AND DISCLOSURES
During this work, the PhD of F. Combes was sponsored by a Convention Industrielle de Formation par la Recherche (CIFRE) from the French government and the Institut Roche de Recherche et Médecine Translationnelle. The authors thank IFR02 and Dr. Hervé Le Nagard for the use of the Centre de Biomodélisation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 164 kb)
Rights and permissions
About this article
Cite this article
Combes, F.P., Retout, S., Frey, N. et al. Prediction of Shrinkage of Individual Parameters Using the Bayesian Information Matrix in Non-Linear Mixed Effect Models with Evaluation in Pharmacokinetics. Pharm Res 30, 2355–2367 (2013). https://doi.org/10.1007/s11095-013-1079-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1079-3