Abstract
Purpose
Orally-taken pirfenidone (PFD), an idiopathic pulmonary fibrosis drug, often causes severe phototoxicity. Present study aimed to develop a respirable powder formulation for PFD (PFD-RP) to minimize phototoxic risk.
Methods
Photochemical properties of PFD were examined using a reactive oxygen species (ROS) assay and photostability testing. PFD-RP was characterized with a focus on photostability, in vitro inhalation performance, and the efficacy in antigen-sensitized rats. Pharmacokinetic studies were conducted after oral and intratracheal administration of PFD formulations.
Results
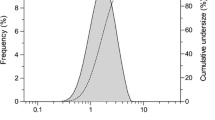
Although PFD solution exhibited photodegradation under simulated sunlight (250 W/m2), both PFD powder and PFD-RP were photochemically stable. Laser diffraction and cascade impactor analyses on PFD-RP suggested its high dispersion and fine in vitro inhalation performance. Inhaled PFD-RP (300 μg-PFD/rat) could suppress antigen-evoked pulmonary inflammation in rats as evidenced by decreases in recruited inflammatory cells and neutrophilia-related biomarkers in the lung. Exposure of PFD to light-exposed tissues (skin and eye) after intratracheal administration of PFD-RP at a pharmacologically effective dose (300 μg-PFD/rat) was 90–130-fold less than that of the oral PFD dosage form at a phototoxic dose (160 mg/kg).
Conclusions
PFD-RP might be an attractive alternative to the current oral PFD therapy with a better safety margin.




Similar content being viewed by others
Abbreviations
- 8-MOP:
-
8-methoxypsoralen
- ANOVA:
-
analysis of variance
- AUC:
-
area under concentration versus time curve
- AUMC:
-
area under moment curve
- BALF:
-
bronchoalveolar lavage fluid
- EPO:
-
eosinophil peroxidase
- ESI-MS:
-
electrospray ionization mass spectrometry
- FQ:
-
fluoroquinolones
- HPMC:
-
hydroxypropyl methylcellulose
- MPO:
-
myeloperoxidase
- MRT:
-
mean residence time
- OVA:
-
ovalbumin
- PBS:
-
phosphate-buffered saline
- ROS:
-
reactive oxygen species
- RP:
-
respirable powder
- SEM:
-
scanning electron microscopy
- TMBZ:
-
3,3′,5,5′-tetramethylbenzidine
- UPLC:
-
ultra performance liquid chromatography
References
Hisatomi K, Mukae H, Sakamoto N, Ishimatsu Y, Kakugawa T, Hara S, et al. Pirfenidone inhibits TGF-ss1-induced over-expression of collagen type I and heat shock protein 47 in A549 cells. BMC Pulm Med. 2012;12:24.
Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999;291:367–73.
Lasky J. Pirfenidone. IDrugs. 2004;7:166–72.
Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011;20:85–97.
Corbel M, Lanchou J, Germain N, Malledant Y, Boichot E, Lagente V. Modulation of airway remodeling-associated mediators by the antifibrotic compound, pirfenidone, and the matrix metalloproteinase inhibitor, batimastat, during acute lung injury in mice. Eur J Pharmacol. 2001;426:113–21.
Hilberg O, Simonsen U, du Bois R, Bendstrup E. Pirfenidone: significant treatment effects in idiopathic pulmonary fibrosis. Clin Respir J. 2012;6:131–43.
Richeldi L, Yasothan U, Kirkpatrick P. Pirfenidone. Nat Rev Drug Discov. 2011;10:489–90.
Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–9.
Carter NJ. Pirfenidone: in idiopathic pulmonary fibrosis. Drugs. 2011;71:1721–1732.
Steinand KR, Scheinfeld NS. Drug-induced photoallergic and phototoxic reactions. Expert Opin Drug Saf. 2007;6:431–43.
Onoue S, Seto Y, Gandy G, Yamada S. Drug-induced phototoxicity; an early in vitro identification of phototoxic potential of new drug entities in drug discovery and development. Curr Drug Saf. 2009;4:123–36.
Seto Y, Inoue R, Ochi M, Gandy G, Yamada S, Onoue S. Combined use of in vitro phototoxic assessments and cassette dosing pharmacokinetic study for phototoxicity characterization of fluoroquinolones. AAPS J. 2011;13:482–92.
Onoue S, Aoki Y, Kawabata Y, Matsui T, Yamamoto K, Sato H, et al. Development of inhalable nanocrystalline solid dispersion of tranilast for airway inflammatory diseases. J Pharm Sci. 2011;100:622–33.
Onoue S, Sato H, Kawabata Y, Mizumoto T, Hashimoto N, Yamada S. In vitro and in vivo characterization on amorphous solid dispersion of cyclosporine A for inhalation therapy. J Control Release. 2009;138:16–23.
Onoueand S, Tsuda Y. Analytical studies on the prediction of photosensitive/phototoxic potential of pharmaceutical substances. Pharm Res. 2006;23:156–64.
Misaka S, Sato H, Yamauchi Y, Onoue S, Yamada S. Novel dry powder formulation of ovalbumin for development of COPD-like animal model: Physicochemical characterization and biomarker profiling in rats. Eur J Pharm Sci. 2009;37:469–76.
Onoue S, Kawamura K, Igarashi N, Zhou Y, Fujikawa M, Yamada H, et al. Reactive oxygen species assay-based risk assessment of drug-induced phototoxicity: Classification criteria and application to drug candidates. J Pharm Biomed Anal. 2008;47:967–72.
Onoue S, Hosoi K, Wakuri S, Iwase Y, Yamamoto T, Matsuoka N et al. Establishment and intra-/inter-laboratory validation of a standard protocol of reactive oxygen species assay for chemical photosafety evaluation. J Appl Toxicol:in press (2012). doi:10.1002/jat.2776.
Onoue S, Takahashi H, Kawabata Y, Seto Y, Hatanaka J, Timmermann B, et al. Formulation design and photochemical studies on nanocrystal solid dispersion of curcumin with improved oral bioavailability. J Pharm Sci. 2010;99:1871–81.
Matsudaand Y, Masahara R. Photostability of solid-state ubidecarenone at ordinary and elevated temperatures under exaggerated UV irradiation. J Pharm Sci. 1983;72:1198–203.
Onoue S, Hashimoto N, Yamada S. Dry powder inhalation systems for pulmonary delivery of therapeutic peptides and proteins. Expert Opin Ther Patents. 2008;18:429–42.
Suarezand S, Hickey AJ. Drug properties affecting aerosol behavior. Respir Care. 2000;45:652–66.
Labirisand NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56:588–99.
Pesci A, Ricchiuti E, Ruggiero R, De Micheli A. Bronchoalveolar lavage in idiopathic pulmonary fibrosis: what does it tell us? Respir Med. 2010;104 Suppl 1:S70–3.
Beeh KM, Beier J, Kornmann O, Buhl R. Neutrophilic inflammation in induced sputum of patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:138–43.
Tzortzaki EG, Tsoumakidou M, Makris D, Siafakas NM. Laboratory markers for COPD in “susceptible” smokers. Clin Chim Acta. 2006;364:124–38.
Onoue S, Misaka S, Kawabata Y, Yamada S. New treatments for chronic obstructive pulmonary disease and viable formulation/device options for inhalation therapy. Expert Opin Drug Deliv. 2009;6:793–811.
Seto Y, Aoki Y, Inoue R, Kojo Y, Kato M, Onoue S, et al. Development of dry powder inhaler system for reducing phototoxic risk. In: Oku N, editor. DDS conference, vol. 20. Shizuoka: Biomedical Research Press; 2011. p. 41–6.
Acknowledgments AND DISCLOSURES
Authors are grateful to Shionogi&Co., Ltd. for kindly providing pirfenidone. This work was supported in part by a Grant-in-Aid for Young Scientists (B) (No. 22790043; S. Onoue) from the Ministry of Education, Culture, Sports, Science, and Technology and a Health Labour Sciences Research Grant from The Ministry of Health, Labour, and Welfare, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Onoue, S., Seto, Y., Kato, M. et al. Inhalable Powder Formulation of Pirfenidone with Reduced Phototoxic Risk for Treatment of Pulmonary Fibrosis. Pharm Res 30, 1586–1596 (2013). https://doi.org/10.1007/s11095-013-0997-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-0997-4