Abstract
Purpose
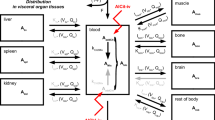
To develop a physiologically based pharmacokinetic (PBPK) model to describe the disposition of Strontium—a bone seeking agent approved in 2004 (as its Ranelate salt) for treatment of osteoporosis in post-menopausal women.
Methods
The model was developed using plasma and bone exposure data obtained from ovariectomised (OVX) female rats—a preclinical model for post-menopausal osteoporosis. The final PBPK model incorporated elements from literature models for bone seeking agents allowing for description of the heterogeneity of bone tissue and also for a physiological description of bone remodelling processes. The model was implemented in MATLAB in open and closed loop configurations, and fittings of the model to exposure data to estimate certain model parameters were carried out using nonlinear regression, treating data with a naïve-pooled approach.
Results
The PBPK model successfully described plasma and bone exposure of Strontium in OVX rats with parameter estimates and model behaviour in keeping with known aspects of the distribution and incorporation of Strontium into bone.
Conclusions
The model describes Strontium exposure in a physiologically rationalized manner and has the potential for future uses in modelling the PK-PD of Strontium, and/or other bone seeking agents, and for scaling to model human Strontium bone exposure.





Similar content being viewed by others
Abbreviations
- Art-blood:
-
Arterial Blood
- Ax :
-
Amount of Strontium in tissue compartment x (mg)
- BFR:
-
Total Bone Formation rate (L/h)
- Clx :
-
Clearance of Strontium from blood by compartment x (L/h)
- Cx :
-
Concentration of Strontium in tissue compartment x (mg/L) equal to Ax/Vx
- FBFR:
-
Fractional Bone Formation Rate
- Fu :
-
Fraction unbound of Strontium in blood
- ka:
-
1st order absorption rate constant for Strontium from gut depot into gut tissue (h−1)
- Kp−x :
-
Tissue to blood partition coefficient for tissue compartment x
- PP:
-
Poorly perfused tissues
- Qx :
-
Blood flow to tissue compartment x (L/h)
- STRONT:
-
Dimensionless scaling factor for Intercompartmental clearance of Strontium from bone tissue surface to bone tissue matrix
- Ven-blood:
-
Mixed Venous blood
- Vx :
-
Volume of tissue compartment x (L)
- WP:
-
Well perfused tissues
- xBFR:
-
Bone Formation rate in bone tissue type x (cortical or trabecular).
- xBRR:
-
Bone Resorption rate in bone tissue type x (cortical or trabecular).
- xRVAF:
-
Intercompartmental clearance of Strontium from bone tissue x surface compartment into bone tissue x matrix compartment (cortical or trabecular).
References
Shorr E, Carter AC. The usefulness of strontium as an adjuvant to calcium in the remineralization of the skeleton in man. Bull Hosp Joint Dis. 1952;13(1):59–66.
McCaslin FE, Janes HM. The effect of strontium lactate in the treatment of osteoporosis. Mayo Clin Proc. 1959;34:329–34.
An YH, RJ. F. Animal Models in Orthopaedic Research. Animal Models in Orthopaedic Research 1998.
Ammann P. Strontium ranelate: a physiological approach for an improved bone quality. Bone. 2006;38(2 Suppl 1):15–8.
Marie PJ, Hott M, Modrowski D, De Pollak C, Guillemain J, Deloffre P, et al. An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. J Bone Miner Res. 1993;8(5):607–15.
Marie PJ, Ammann P, Boivin G, Rey C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif Tissue Int. 2001;69(3):121–9.
Ammann P. Strontium ranelate: a novel mode of action leading to renewed bone quality. Osteoporos Int. 2005;16 Suppl 1:S11–5.
Marie PJ. Strontium ranelate: a novel mode of action optimizing bone formation and resorption. Osteoporos Int. 2005;16 Suppl 1:S7–S10.
Grynpas MD, Hamilton E, Cheung R, Tsouderos Y, Deloffre P, Hott M, et al. Strontium increases vertebral bone volume in rats at a low dose that does not induce detectable mineralization defect. Bone. 1996;18(3):253–9.
Busse B, Jobke B, Hahn M, Priemel M, Niecke M, Seitz S, et al. Effects of strontium ranelate administration on bisphosphonate-altered hydroxyapatite: Matrix incorporation of strontium is accompanied by changes in mineralization and microstructure. Acta Biomater. 2010;6(12):4513–21.
Jobke B, Burghardt AJ, Muche B, Hahn M, Semler J, Amling M, et al. Trabecular reorganization in consecutive iliac crest biopsies when switching from bisphosphonate to strontium ranelate treatment. PLoS One. 2011;6(8):e23638.
Canalis E, Hott M, Deloffre P, Tsouderos Y, Marie PJ. The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitro. Bone. 1996;18(6):517–23.
Takahashi N, Sasaki T, Tsouderos Y, Suda T. S 12911–2 inhibits osteoclastic bone resorption in vitro. J Bone Miner Res. 2003;18(6):1082–7.
Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350(5):459–68.
Reginster JY, Seeman E, De Vernejoul MC, Adami S, Compston J, Phenekos C, et al. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90(5):2816–22.
Reginster JY, Kaufman JM, Goemaere S, Devogelaer JP, Benhamou CL, Felsenberg D, et al. Maintenance of antifracture efficacy over 10 years with strontium ranelate in postmenopausal osteoporosis. Osteoporos Int. 2011 Nov 29.
Stepensky D, Kleinberg L, Hoffman A. Bone as an effect compartment: models for uptake and release of drugs. Clin Pharmacokinet. 2003;42(10):863–81.
Pors NS. The biological role of strontium. Bone. 2004;35(3):583–8.
Dahl SG, Allain P, Marie PJ, Mauras Y, Boivin G, Ammann P, et al. Incorporation and distribution of strontium in bone. Bone. 2001;28(4):446–53.
Marie PJ. Strontium as therapy for osteoporosis. Curr Opin Pharmacol. 2005;5(6):633–6.
Apostoaei AI. Absorption of strontium from the gastrointestinal tract into plasma in healthy human adults. Health Phys. 2002;83(1):56–65.
Moraes ME, Aronson JK, Grahame-Smith DG. Intravenous strontium gluconate as a kinetic marker for calcium in healthy volunteers. Br J Clin Pharmacol. 1991;31(4):423–7.
Samachson J. The gastrointestinal clearance of Strontium-85 and calcium-45 in man. Radiat Res. 1966;27(1):64–74.
Samachson J, Spencer-Laszlo H. Urinary excretion of calcium and Strontium-85 in man. J Appl Phys. 1962;17:525–30.
Walser M. Renal Excretion of Alkaline Earths. In: Comar CL, Bronner F, editors. Mineral Metabolism. An Advanced Treatise. Chapter IV. New York: Academic; 1969. p. 235–320.
Mundy GR, Martin TJ. Physiology and pharmacology of bone. Physiology and pharmacology of bone: Handbook of experimental pharmacology; v 107. 1993:xxv, 762.
O’Flaherty EJ. Physiologically based models of metal kinetics. Crit Rev Toxicol. 1998;28(3):271–317.
Staub JF, Foos E, Courtin B, Jochemsen R, Perault-Staub AM. A nonlinear compartmental model of Sr metabolism. I. Non-steady-state kinetics and model building. Am J Physiol Regul Integr Comp Physiol. 2003;284(3):R819–34.
Staub JF, Foos E, Courtin B, Jochemsen R, Perault-Staub AM. A nonlinear compartmental model of Sr metabolism. II. Its physiological relevance for Ca metabolism. Am J Physiol Regul Integr Comp Physiol. 2003;284(3):R835–52.
Leggett RW. A generic age-specific biokinetic model for calcium-like elements. Radiat Prot Dosim. 1992;41(2–4):183–98.
Leeuwenkamp OR, van der Vijgh WJ, Husken BC, Lips P, Netelenbos JC. Quantification of strontium in plasma and urine with flameless atomic absorption spectrometry. Clin Chem. 1989;35(9):1911–4.
D’Haese PC, Van Landeghem GF, Lamberts LV, Bekaert VA, Schrooten I, De Broe ME. Measurement of strontium in serum, urine, bone, and soft tissues by Zeeman atomic absorption spectrometry. Clin Chem. 1997;43(1):121–8.
Barto R, Sips AJ, van der Vijgh WJ, Netelenbos JC. Sensitive method for analysis of strontium in human and animal plasma by graphite furnace atomic absorption spectrophotometry. Clin Chem. 1995;41(8 Pt 1):1159–63.
Nestorov I. Whole body pharmacokinetic models. Clin Pharmacokinet. 2003;42(10):883–908.
O’Flaherty EJ. Physiologically based models for bone-seeking elements. I. Rat skeletal and bone growth. Toxicol Appl Pharmacol. 1991;111(2):299–312.
O’Flaherty EJ. Physiologically based models for bone-seeking elements. II. Kinetics of lead disposition in rats. Toxicol Appl Pharmacol. 1991;111(2):313–31.
Zucker TF. The Growth Curve of the albino rat in relation to diet. J Nutr. 1941.
Mundy GR. Cellular and molecular regulation of bone turnover. Bone. 1999;24(5 Suppl):35S–8S.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–5.
Jones HM, Parrott N, Jorga K, Lave T. A novel strategy for physiologically based predictions of human pharmacokinetics. Clin Pharmacokinet. 2006;45(5):511–42.
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13(4):407–84.
Nestorov IA, Aarons LJ, Arundel PA, Rowland M. Lumping of whole-body physiologically based pharmacokinetic models. J Pharmacokinet Biopharm. 1998;26(1):21–46.
Landaw EM, DiStefano 3rd JJ. Multiexponential, multicompartmental, and noncompartmental modeling. II. Data analysis and statistical considerations. Am J Physiol. 1984;246(5 Pt 2):R665–77.
Ette EI, Williams PJ. Population pharmacokinetics II: estimation methods. Ann Pharmacother. 2004;38(11):1907–15.
Gibaldi M, Perrier D. Pharmacokinetics (2nd ed.). 1982.
Comar CL, Georgi J. Assessment of chronic exposure to radiostrontium by urinary assay. Nature. 1961;191:390–1.
Comar CL, Nold MM, Wasserman RH. Strontium-calcium discrimination factors in the rat. Proc Soc Exp Biol Med. 1956;92(4):859–63.
Aarons L. Physiologically based pharmacokinetic modelling: a sound mechanistic basis is needed. Br J Clin Pharmacol. 2005;60(6):581–3.
O’Flaherty EJ. Physiologically based models for bone-seeking elements. III. Human skeletal and bone growth. Toxicol Appl Pharmacol. 1991;111(2):332–41.
O’Flaherty EJ. Physiologically based models for bone-seeking elements. IV. Kinetics of lead disposition in humans. Toxicol Appl Pharmacol. 1993;118(1):16–29.
Lemaire V, Tobin FL, Greller LD, Cho CR, Suva LJ. Modeling the interactions between osteoblast and osteoclast activities in bone remodeling. J Theor Biol. 2004;229(3):293–309.
Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, et al. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278(50):50259–72.
Cox EH, Veyrat-Follet C, Beal SL, Fuseau E, Kenkare S, Sheiner LB. A population pharmacokinetic-pharmacodynamic analysis of repeated measures time-to-event pharmacodynamic responses: the antiemetic effect of ondansetron. J Pharmacokinet Biopharm. 1999;27(6):625–44.
Acknowledgments and Disclosures
The authors wish to thank Dr Kayode Ogungbenro and Dr Aris Dokoutmetzidis for their useful comments and assistance with Matlab during model development.
The authors would like also to acknowledge Dr Isabelle Dupin-Roger, Dr Pascal Delrat and Dr Emmanuelle Foos-Gilbert for their useful comments on Strontium pharmacology and Strontium ranelate pharmacokinetics in rat.
Work in this paper was funded by Servier research and development and by the School of Pharmacy and Pharmaceutical Sciences, The University of Manchester.
Part of this work was presented by the authors at the 20th Population Approach Group Europe (PAGE) meeting in Athens, Greece, June 7–10, 2011.
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Appendix 1
Tables of tissue concentrations and tissue-to-blood ratios from tissue distribution study.
Rights and permissions
About this article
Cite this article
Pertinez, H., Chenel, M. & Aarons, L. A Physiologically Based Pharmacokinetic Model for Strontium Exposure in Rat. Pharm Res 30, 1536–1552 (2013). https://doi.org/10.1007/s11095-013-0991-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-0991-x