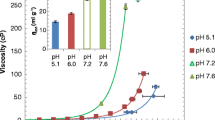
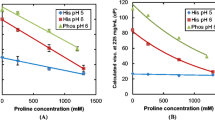
ABSTRACT
Purpose
To discover, elucidate the structure-activity relationship (SAR), and explore the mechanism of action of excipients able to drastically lower the viscosities of concentrated aqueous solutions of humanized monoclonal antibodies (MAbs).
Methods
Salts prepared from hydrophobic cations and anions were dissolved into humanized MAbs solutions. Viscosities of the resulting solutions were measured as a function of the nature and concentration of the salts and MAbs.
Results
Even at moderate concentrations, some of the salts prepared herein were found to reduce over 10-fold the viscosities of concentrated aqueous solutions of several MAbs at room temperature.
Conclusions
To be potent viscosity-lowering excipients, the ionic constituents of the salts must be hydrophobic, bulky, and aliphatic. A mechanistic hypothesis explaining the observed salt effects on MAb solutions’ viscosities was proposed and verified.






Similar content being viewed by others
Abbreviations
- CHO:
-
Chinese hamster ovary
- MAb:
-
monoclonal antibody
- SAR:
-
structure-activity relationship
- SC:
-
subcutaneous
- SD:
-
standard deviation
REFERENCES
Yang MX, Shenoy B, Disttler M, Patel R, McGrath M, Pechenov S, Margolin AL. Crystalline monoclonal antibodies for subcutaneous delivery. Proc Natl Acad Sci USA. 2003;100(12):6934–9.
Shire SJ, Shahrokh Z, Liu J. Challenges in the development of high protein concentration formulations. J Pharm Sci. 2004;93(6):1390–402.
Liu J, Nguen MDH, Andya JD, Shire SJ. Reversible self-association increases the viscosity of a concentrated monoclonal antibody in aqueous solution. J Pharm Sci. 2005;94(9):1928–40.
Kanai S, Liu J, Patapoff HW, Shire SJ. Reversible self-association of a concentrated monoclonal antibody solution mediated by Fab-Fab interaction that impacts solution viscosity. J Pharm Sci. 2008;97(10):4219–27.
Du W, Klibanov AM. Hydrophobic salts markedly diminish viscosity of concentrated protein solutions. Biotechnol Bioeng. 2011;108(3):632–6.
He F, Becker GW, Litowski JR, Narhi LO, Brems DN, Razinkov VI. High throughput dynamic light scattering method for measuring viscosity of concentrated protein solutions. Anal Biochem. 2010;399(1):141–3.
Papanagopoulos D, Dondos A. Difference between the dynamic and static behavior of polymers in dilute solutions: The critical concentrations. Polymer. 1995;36(2):369–72.
Saluja A, Kalonia DS. Nature and consequences of protein–protein interactions in high protein concentration solutions. Int J Pharm. 2008;358(1–2):1–15.
Harris RJ, Shire SJ, Winter C. Commercial manufacturing scale formulation and analytical characterization of therapeutic recombinant antibodies. Drug Dev Res. 2004;61(3):137–54.
Frokjaer S, Otzen DE. Protein drug stability: a formulation challenge. Nat Rev Drug Discov. 2005;4:298–306.
Paulekuhn GS, Dressman JB, Saal C. Trends in active pharmaceutical ingredient salt selection based on analysis of the Orange Book database. J Med Chem. 2007;50(26):6665–72.
Yadav S, Shire SJ, Kalonia DS. Factors affecting the viscosity in high concentration solutions of different monoclonal antibodies. J Pharm Sci. 2010;99(12):4812–29.
Burckbuchler V, Mekhloufi G, Giteau AP, Grossiord JL, Huille S, Agnely F. Rheological and syringeability properties of highly concentrated human polyclonal immunoglobulin solutions. Eur J Pharm Biopharm. 2010;76(3):351–6.
Tung MA. Rheology of proteins dispersions. J Texture Stud. 1978;9(1–2):3–31.
Hall CG, Abraham GN. Reversible self-association of a human myeloma protein. Thermodynamics and relevance to viscosity effects and solubility. Biochemistry. 1984;23(22):5123–9.
Kinsella JE. Milk proteins: physicochemical and functional properties. Crit Rev Food Sci Nutr. 1984;21(3):197–262.
Lee C-H, Rha C. In: Sherman P, editor. Food texture and rheology. London: Academic; 1979. p. 245–63.
Sharma V, Jaishankar A, Wang Y-C, McKinley GH. Rheology of globular proteins: apparent yield stress, high shear rate viscosity and interfacial viscoelasticity of bovine serum albumin solutions. Soft Matter. 2011;7(11):5150–60.
Patapoff TW, Esue O. Polysorbate 20 prevents the precipitation of a monoclonal antibody during shear. Pharm Dev Technol. 2009;14(6):659–64.
ACKNOWLEDGMENTS AND DISCLOSURES
This study was financially supported by Novartis AG, which also kindly supplied the humanized MAbs used by us.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, Z., Chen, A., Nassar, R.A. et al. Structure-Activity Relationship for Hydrophobic Salts as Viscosity-Lowering Excipients for Concentrated Solutions of Monoclonal Antibodies. Pharm Res 29, 3102–3109 (2012). https://doi.org/10.1007/s11095-012-0802-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0802-9