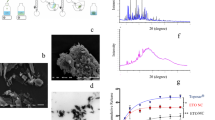
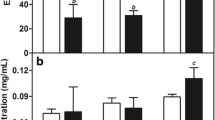
Abstract
Purpose
To develop a nanocrystalline paclitaxel formulation with a high paclitaxel-to-stabilizer ratio which can be used for hyperthermic intraperitoneal chemotherapy (HIPEC).
Methods
Paclitaxel (PTX) nanocrystals were prepared via wet milling using Pluronic F127® as stabilizer. The suitability of paclitaxel nanosuspensions for HIPEC treatment was evaluated by analyzing the cytotoxicity of both stabilizer and formulation, and by determining the maximum tolerated dose (MTD) and bioavailability. The effect on tumor growth was evaluated by magnetic resonance imaging (MRI) at day 7 and 14 after HIPEC treatment in rats with peritoneal carcinomatosis of ovarian origin.
Results
Monodisperse nanosuspensions (±400 nm) were developed using Pluronic F127® as single additive. The cytotoxicity and MTD of this nanocrystalline formulation was similar compared to Taxol®, while its bioavailability was higher. MRI data after HIPEC treatment with a PTX nanocrystalline suspension showed a significant reduction of tumor volume compared to the non-treated group. Although no significant differences on tumor volume were observed between Taxol® and the nanosuspension, the rats treated with the nanosuspension recovered faster following the HIPEC procedure.
Conclusion
Nanosuspensions with a high paclitaxel-to-stabilizer ratio are of interest for the treatment of peritoneal carcinomatosis of ovarian origin via HIPEC.







Similar content being viewed by others
Abbreviations
- HIPEC:
-
hyperthermic intraperitoneal chemotherapy
- MTD:
-
maximum tolerated dose
- PEO:
-
polyethylene oxide
- Plu F127:
-
Pluronic F127®
- Plu F68:
-
Pluronic F68®
- PPO:
-
polypropylene oxide
- PTX:
-
paclitaxel
- TGD:
-
tumor growth delay
References
Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46(4):765–81.
Sugarbaker PH. Comprehensive management of peritoneal surface malignancy using cytoreductive surgery and perioperative intraperitoneal chemotherapy: the Washington Cancer Institute approach. Expert Opin Pharmacother. 2009;10(12):1965–77.
Mohamed F, Cecil T, Moran B, Sugarbaker P. A new standard of care for the management of peritoneal surface malignancy. Curr Oncol. 2011;18(2):E84–96.
Bristow RE, Puri I, Chi DS. Cytoreductive surgery for recurrent ovarian cancer: A meta-analysis. Gynecol Oncol. 2009;112(1):265–74.
Dovern E, de Hingh I, Verwaal VJ, van Driel WJ, Nienhuijs SW. Hyperthermic intraperitoneal chemotherapy added to the treatment of ovarian cancer. A review of achieved results and complications. Eur J Gynaecol Oncol. 2010;31(3):256–61.
Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4(5):277–83.
Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590–8.
Colson YL, Liu R, Southard EB, Schulz MD, Wade JE, Griset AP, et al. The performance of expansile nanoparticles in a murine model of peritoneal carcinomatosis. Biomaterials. 2011;32(3):832–40.
Peltonen L, Hirvonen J. Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods. J Pharm Pharmacol. 2010;62(11):1569–79.
Kesisoglou F, Panmai S, Wu Y. Application of nanoparticles in oral delivery of immediate release formulations. Current Nanoscience. 2007;3(2):183–90.
Liu F, Park J-Y, Zhang Y, Conwell C, Liu Y, Bathula SR, et al. Targeted Cancer Therapy With Novel High Drug-Loading Nanocrystals. J Pharm Sci. 2010;99(8):3542–51.
Yeo Y, Ito T, Bellas E, Highley CB, Marini R, Kohane DS. In situ cross-linkable hyaluronan hydrogels containing polymeric nanoparticles for preventing postsurgical adhesions. Ann Surg. 2007;245(5):819–24.
Bouquet W, Ceelen W, Adriaens E, Almeida A, Quinten T, De Vos F, et al. In vivo Toxicity and Bioavailability of Taxol (R) and a Paclitaxel/beta-Cyclodextrin Formulation in a Rat Model During HIPEC. Ann Surg Oncol. 2010;17(9):2510–7.
Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23(12):2709–28.
Kabanov AV, Batrakova EV, Alakhov VY. Pluronic((R)) block copolymers for overcoming drug resistance in cancer. Adv Drug Deliv Rev. 2002;54(5):759–79.
Batrakova EV, Li S, Brynskikh AM, Sharma AK, Li YL, Boska M, et al. Effects of pluronic and doxorubicin on drug uptake, cellular metabolism, apoptosis and tumor inhibition in animal models of MDR cancers. J Control Release. 2010;143(3):290–301.
Merisko-Liversidge E, Liversidge GG. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv Drug Deliv Rev. 2011;63(6):427–40.
Ghosh I, Bose S, Vippagunta R, Harmon F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int J Pharm. 2011;409(1–2):260–8.
Debree E, Rosing H, Filis D, Romanos J, Melisssourgaki M, Daskalakis M, et al. Cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy with paclitaxel: A clinical and pharmacokinetic study. Ann Surg Oncol. 2008;15(4):1183–92.
Bajaj G, Yeo Y. Drug delivery systems for intraperitoneal therapy. Pharm Res. 2010;27(5):735–8.
Liu P, Rong XY, Laru J, van Veen B, Kiesvaara J, Hirvonen J, et al. Nanosuspensions of poorly soluble drugs: Preparation and development by wet milling. Int J Pharm. 2011;411(1–2):215–22.
Tannock IF, Lee CM, Tunggal JK, Cowan DSM, Egorin MJ. Limited penetration of anticancer drugs through tumor tissue: A potential cause of resistance of solid tumors to chemotherapy. Clin Cancer Res. 2002;8(3):878–84.
Fujiwara K, Armstrong D, Morgan M, Markman M. Principles and practice of intraperitoneal chemotherapy for ovarian cancer. Int J Gynecol Cancer. 2007;17(1):1–20.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Smet, L., Colin, P., Ceelen, W. et al. Development of a Nanocrystalline Paclitaxel Formulation for Hipec Treatment. Pharm Res 29, 2398–2406 (2012). https://doi.org/10.1007/s11095-012-0765-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0765-x