ABSTRACT
Purpose
To effectively inhibit succinate buffer crystallization and the consequent pH changes in frozen solutions.
Methods
Using differential scanning calorimetry (DSC) and X-ray diffractometry (XRD), the crystallization behavior of succinate buffer in the presence of either (i) a crystallizing (glycine, mannitol, trehalose) or (ii) a non-crystallizing cosolute (sucrose) was evaluated. Aqueous succinate buffer solutions, 50 or 200 mM, at pH values 4.0 or 6.0 were cooled from room temperature to −25°C at 0.5°C/min. The pH of the solution was measured as a function of temperature using a probe designed to function at low temperatures. The final lyophiles prepared from these solutions were characterized using synchrotron radiation.
Results
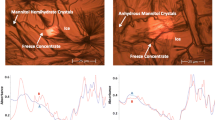
When the succinic acid solution buffered to pH 4.0, in the absence of a cosolute, was cooled, there was a pronounced shift in the freeze-concentrate pH. Glycine and mannitol, which have a tendency to crystallize in frozen solutions, remained amorphous when the initial pH was 6.0. Under this condition, they also inhibited buffer crystallization and prevented pH change. At pH 4.0 (50 mM initial concentration), glycine and mannitol crystallized and did not prevent pH change in frozen solutions. While sucrose, a non-crystallizing cosolute, did not completely prevent buffer crystallization, the extent of crystallization was reduced. Sucrose decomposition, based on XRD peaks attributable to β-D-glucose, was observed in frozen buffer solutions with an initial pH of 4.0. Trehalose completely inhibited crystallization of the buffer components when the initial pH was 6.0 but not at pH 4.0. At the lower pH, the crystallization of both trehalose dihydrate and buffer components was evident.
Conclusion
When retained amorphous, sucrose and trehalose effectively inhibited succinate buffer component crystallization and the consequent pH shift. However, when trehalose crystallized or sucrose degraded to yield a crystalline decomposition product, crystallization of buffer was observed. Similarly, glycine and mannitol, two widely used bulking agents, inhibited buffer component crystallization only when retained amorphous. In addition to stabilizing the active pharmaceutical ingredient, lyoprotectants may prevent solution pH shift by inhibiting buffer crystallization.











Similar content being viewed by others
REFERENCES
Akers MJ, Defelippis MR. Peptides and proteins as parenteral solutions. In: Frokjaer S, Hovgaard L, editors. Pharmaceutical Formulation Development of Peptide and Proteins. Philadelphia: Taylor and Francis Inc.; 2003. p. 145–77.
Akers MJ, Fites AL, Robison RL. Formulation design and development of parenteral suspensions. J Parenter Sci Technol. 1987;41:88–96.
Akers MJ, Vasudevan V, Stickelmeyer M. Formulation development of protein dosage forms. In: Nail SL, Akers MJ, editors. Development and manufacture of protein pharmaceuticals. New York: Kluwer Academic/Plenum publishers; 2002. p. 47–127.
Defelippis MR, Akers MJ. Peptides and proteins as parenteral suspensions: An overview of design, development, and manufacturing considerations. In: Frokjaer S, Hovgaard L, editors. Pharmaceutical Formulation Development of Peptide and Proteins. Philadelphia: Taylor and Francis Inc; 2000. p. 113. 2003.
Pikal MJ. Freeze drying. In: Swarbrick J, editor. Encyclopedia of pharmaceutical technology, vol. 1. New York: Informa Healthcare; 2007. p. 1807–33.
Trissel LA. Handbook on injectable drugs. 14th ed. Bethesda: American Society of Health-System Pharmacists; 2007.
Shalaev EY. The impact of buffer on processing and stability of freeze-dried dosage forms, part 1: Solution freezing behavior. Am Pharm Rev. 2005;8:80–7.
Shalaev EY, Johnson-Elton TD, Chang L, Pikal MJ. Thermophysical properties of pharmaceutically compatible buffers at sub-zero temperatures: Implications for freeze-drying. Pharm Res. 2002;19:195–201.
van den Berg L. pH changes in buffers and foods during freezing and subsequent storage. Cryobiology. 1966;3:236–42.
van den Berg L, Rose D. Effect of freezing on the pH and composition of sodium and potassium phosphate solutions: The reciprocal system KH2PO4-Na2HPO4-H2O. Arch Biochem Biophys. 1959;81:319–29.
Varshney DB, Sundaramurthi P, Shalaev EY, Kumar S, Kang S-W, Gatlin LA, et al. Phase transitions in frozen systems and during freeze-drying: Quantification using synchrotron X-ray diffractometry. Pharm Res. 2009;26:1064–75.
Pikal-Cleland KA, Cleland JL, Anchordoquy TJ, Carpenter JF. Effect of glycine on pH changes and protein stability during freeze-thawing in phosphate buffer systems. J Pharm Sci. 2002;91:1969–79.
Akers MJ. Excipient—drug interactions in parenteral formulations. J Pharm Sci. 2002;91:2283–300.
Sundaramurthi P, Shalaev E, Suryanarayanan R. "pH swing" in frozen solutions-consequence of sequential crystallization of buffer components. J Phys Chem Lett. 2010;1:265–8.
Sundaramurthi P, Shalaev E, Suryanarayanan R. Calorimetric and diffractometric evidence for the sequential crystallization of buffer components and consequent pH swing in frozen solutions. J Phys Chem B. 2010;114:4915–23.
Sundaramurthi P, Suryanarayanan R. Trehalose crystallization during freeze-drying: Implications on lyoprotection. J Phys Chem Lett. 2010;1:510–4.
Varshney DB, Kumar S, Shalaev EY, Sundaramurthi P, Kang S-W, Gatlin LA, et al. Glycine crystallization in frozen and freeze-dried systems: Effect of pH and buffer concentration. Pharm Res. 2007;24:593–604.
Sundaramurthi P, Patapoff TW, Suryanarayanan R. Crystallization of trehalose in frozen solutions and its phase behavior during drying. Pharm. Res. 2010, doi:10.1007/s111095-010-0243-2.
Yalkowsky SH, He Y. Handbook of aqueous solubility data. New York: CRC press; 2003.
Chongprasert S, Knopp SA, Nail SL. Characterization of frozen solutions of glycine. J Pharm Sci. 2001;90:1720–8.
Kasraian K, Spitznagel TM, Juneau JA, Yim K. Characterization of the sucrose/glycine/water system by differential scanning calorimetry and freeze-drying microscopy. Pharm Dev Technol. 1998;3:233–9.
Li X, Nail SL. Kinetics of glycine crystallization during freezing of sucrose/glycine excipient systems. J Pharm Sci. 2005;94:625–31.
Pyne A, Suryanarayanan R. Phase transitions of glycine in frozen aqueous solutions and during freeze-drying. Pharm Res. 2001;18:1448–54.
Yu L, Ng K. Glycine crystallization during spray-drying: The pH effect on salt and polymorphic forms. J Pharm Sci. 2002;91:2367–75.
Chang BS, Randall CS. Use of subambient thermal analysis to optimize protein lyophilization. Cryobiology. 1992;29:632–56.
Sundaramurthi P, Suryanarayanan R. Influence of crystallizing and non-crystallizing cosolutes on trehalose crystallization during freeze-drying. Pharm. Res. 2010, doi:10.1007/s111095-010-0221-8.
Pikal-Cleland KA, Rodriguez-Hornedo N, Amidon GL, Carpenter JF. Protein denaturation during freezing and thawing in phosphate buffer systems: Monomeric and tetrameric beta -galactosidase. Arch Biochem Biophys. 2000;384:398–406.
Sundaramurthi P, Suryanarayanan R. Predicting the crystallization propensity of carboxylic acid buffers in frozen systems—relevance to freeze-drying J. Pharm. Sci. 2010, in press.
Telang C, Yu L, Suryanarayanan R. Effective inhibition of mannitol crystallization in frozen solutions by sodium chloride. Pharm Res. 2003;20:660–7.
Telang C, Suryanarayanan R, Yu L. Crystallization of D-mannitol in binary mixtures with NaCl: Phase diagram and polymorphism. Pharm Res. 2003;20:1939–45.
Izutsu K-i, Ocheda SO, Aoyagi N, Kojima S. Effects of sodium tetraborate and boric acid on nonisothermal mannitol crystallization in frozen solutions and freeze-dried solids. Int J Pharm. 2004;273:85–93.
Izutsu K-i, Yomota C, Aoyagi N. Inhibition of mannitol crystallization in frozen solutions by sodium phosphates and citrates. Chem Pharm Bull. 2007;55:565–70.
Powder Diffraction File. Hexagonal ice, card # 00-042-1142; D- trehalose dihydrate, card # 00-029-1955; β-succinic acid, card # 00-031-1899; monosodium succinate, card # 00-030-1927; sucrose, card # 00-024-1977; β-D-Glucose 00-039-1837; β-D-mannitol, card #00-022-1797; δ-D-mannitol, card # 00-022-1794. International Centre for Diffraction Data, Newtown Square, PA; 2004.
ACKNOWLEDGEMENTS
The XRD studies were carried out in the College of Science and Engineering Characterization Facility, University of Minnesota, which receives partial support from NSF through the MRSEC program. The use of the Advanced Photon Source at Argonne National Laboratory through the Midwest Universities Collaborative Access Team (MUCAT sector) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sundaramurthi, P., Suryanarayanan, R. The Effect of Crystallizing and Non-crystallizing Cosolutes on Succinate Buffer Crystallization and the Consequent pH Shift in Frozen Solutions. Pharm Res 28, 374–385 (2011). https://doi.org/10.1007/s11095-010-0282-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0282-8