ABSTRACT
Purpose
The objective of this investigation was to assess whether common pharmaceutical excipients regulate the expression of drug-metabolizing enzymes in human colon and liver cells.
Methods
Nineteen commonly used excipients were evaluated using a panel of experiments including cell-based human PXR activation assays, real-time RT-PCR assays for CYP3A4 mRNA expression, and immunoblot analysis of CYP3A4 protein expression in immortalized human liver cells (HepG2 and Fa2N4), human primary hepatocytes, and the intestinal LS174T cell models.
Results
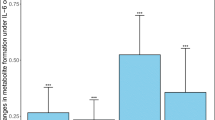
No excipient activated human PXR or practically induced CYP3A4. However, three excipients (polysorbate 80, pregelatinized starch, and hydroxypropyl methylcellulose) tended to decrease mRNA and protein expression across experimental models.
Conclusion
This study represents the first investigation of the potential role of excipients in the expression of drug-metabolizing enzymes. Findings imply that some excipients may hold potential for excipient-drug interactions by repression of CYP3A4 expression.




Similar content being viewed by others
Abbreviations
- CA:
-
Citric acid
- CCS:
-
croscarmellose sodium
- CYP3A4:
-
cytochrome P450 3A4
- DCP:
-
dicalcium phosphate dehydrate
- DMSO:
-
dimethyl sulfoxide
- FA:
-
fumaric acid
- HPMC:
-
hydroxypropyl methylcellulose
- KTC:
-
ketaconazole
- LAC:
-
lactose
- MA:
-
malic acid
- MCC:
-
microcrystalline cellulose
- MgStr:
-
magnesium stearate
- MDR1:
-
multidrug resistance
- PEG-3350:
-
polyethylene glycol 3350
- PS-80:
-
polysorbate-80
- PVP:
-
povidone
- PXR:
-
pregnane X receptor
- PgS:
-
pregelatinized starch
- PG:
-
propylene glycol
- RIF:
-
rifampicin
- SD:
-
amorphous fumed silicon dioxide
- SLS:
-
sodium lauryl sulfate
- SSG:
-
sodium starch glycolate
- SUC:
-
sucrose
- X-PVP:
-
Crospovidone
REFERENCES
FDA. Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. CDER, Guidance for Industry. 2000.
EMEA. Note for guidance on the investigation of bioavailability and bioequivalence. Committee for Proprietary Medicinal Products. 2001.
Polli JE, Yu LX, Cook JA, Amidon GL, Borchardt RT, Burnside BA, et al. Summary workshop report: biopharmaceutics classification system–implementation challenges and extension opportunities. J Pharm Sci. 2004;93:1375–81.
Polli JE, Abrahamsson BS, Yu LX, Amidon GL, Baldoni JM, Cook JA, et al. Summary workshop report: bioequivalence, biopharmaceutics classification system, and beyond. AAPS J. 2008;10:373–9.
Yu LX, Amidon GL, Polli JE, Zhao H, Mehta MU, Conner DP, et al. Biopharmaceutics classification system: the scientific basis for biowaiver extensions. Pharm Res. 2002;19:921–5.
Benet LZ, Amidon GL, Barends DM, Lennernas H, Polli JE, Shah VP, et al. The use of BDDCS in classifying the permeability of marketed drugs. Pharm Res. 2008;25:483–8.
EMEA. Guideline on the investigation of bioequivalence. Committee for Medicinal Products for Human Use. 2008.
Koch KM, Parr AF, Tomlinson JJ, Sandefer EP, Digenis GA, Donn KH, et al. Effect of sodium acid pyrophosphate on ranitidine bioavailability and gastrointestinal transit time. Pharm Res. 1993;10:1027–30.
Adkin DA, Davis SS, Sparrow RA, Huckle PD, Wilding IR. The effect of mannitol on the oral bioavailability of cimetidine. J Pharm Sci. 1995;84:1405–9.
Wuand CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23.
Klaassen CD, Slitt AL. Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab. 2005;6:309–28.
Meyer UA. Overview of enzymes of drug metabolism. J Pharmacokinet Biopharm. 1996;24:449–59.
Lin JH. Sense and nonsense in the prediction of drug-drug interactions. Curr Drug Metab. 2000;1:305–31.
Wang H, LeCluyse EL. Role of orphan nuclear receptors in the regulation of drug-metabolising enzymes. Clin Pharmacokinet. 2003;42:1331–57.
Sachs-Barrable K, Thamboo A, Lee SD, Wasan KM. Lipid excipients Peceol and Gelucire 44/14 decrease P-glycoprotein mediated efflux of rhodamine 123 partially due to modifying P-glycoprotein protein expression within Caco-2 cells. J Pharm Pharm Sci. 2007;10:319–31.
Barta CA, Sachs-Barrable K, Feng F, Wasan KM. Effects of monoglycerides on P-glycoprotein: modulation of the activity and expression in Caco-2 cell monolayers. Mol Pharm. 2008;5:863–75.
Kumar GN, Surapaneni S. Role of drug metabolism in drug discovery and development. Med Res Rev. 2001;21:397–411.
Wang H, Tompkins LM. CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme. Curr Drug Metab. 2008;9:598–610.
Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82.
Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Investig. 1998;102:1016–23.
LeCluyse EL, Alexandre E, Hamilton GA, Viollon-Abadie C, Coon DJ, Jolley S, et al. Isolation and culture of primary human hepatocytes. Methods Mol Biol (Clifton, NJ). 2005;290:207–29.
Li L, Chen T, Stanton JD, Sueyoshi T, Negishi M, Wang H. The peripheral benzodiazepine receptor ligand 1-(2-chlorophenyl-methylpropyl)-3-isoquinoline-carboxamide is a novel antagonist of human constitutive androstane receptor. Mol Pharmacol. 2008;74:443–53.
Faucette SR, Sueyoshi T, Smith CM, Negishi M, Lecluyse EL, Wang H. Differential regulation of hepatic CYP2B6 and CYP3A4 genes by constitutive androstane receptor but not pregnane X receptor. J Pharmacol Exp Ther. 2006;317:1200–9.
Wang H, Faucette S, Sueyoshi T, Moore R, Ferguson S, Negishi M, et al. A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J Biol Chem. 2003;278:14146–52.
Li L, Stanton JD, Tolson AH, Luo Y, Wang H. Bioactive terpenoids and flavonoids from Ginkgo biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor-mediated pathways. Pharm Res. 2009;26:872–82.
Habib YS, Augsburger LL, Shangraw RF. Production of inert cushioning beads: effect of excipients on the physicomechanical properties of freeze-dried beads containing microcrystalline cellulose produced by extrusion-spheronization. Int J Pharm. 2002;233:67–83.
Shangraw RF. Emerging trends in the use of pharmaceutical excipients. Pharm Technol. 1997;21:36–42.
Digenis GA, Gold TB, Shah VP. Cross-linking of gelatin capsules and its relevance to their in vitro-in vivo performance. J Pharm Sci. 1994;83:915–21.
Fetzner A, Bohm S, Schreder S, Schubert R. Degradation of raw or film-incorporated beta-cyclodextrin by enzymes and colonic bacteria. Eur J Pharm Biopharm. 2004;58:91–7.
Yamagata T, Kusuhara H, Morishita M, Takayama K, Benameur H, Sugiyama Y. Improvement of the oral drug absorption of topotecan through the inhibition of intestinal xenobiotic efflux transporter, breast cancer resistance protein, by excipients. Drug Metab Dispos. 2007;35:1142–8.
Yamagata T, Morishita M, Kusuhara H, Takayama K, Benameur H, Sugiyama Y. Characterization of the inhibition of breast cancer resistance protein-mediated efflux of mitoxantrone by pharmaceutical excipients. Int J Pharm. 2009;370:216–9.
Luo G, Cunningham M, Kim S, Burn T, Lin J, Sinz M, et al. CYP3A4 induction by drugs: correlation between a pregnane X receptor reporter gene assay and CYP3A4 expression in human hepatocytes. Drug Metab Dispos. 2002;30:795–804.
FDA. In vitro evaluation of CYP induction. FDA Guidelines. 2006.
Burk O, Koch I, Raucy J, Hustert E, Eichelbaum M, Brockmoller J, et al. The induction of cytochrome P450 3A5 (CYP3A5) in the human liver and intestine is mediated by the xenobiotic sensors pregnane X receptor (PXR) and constitutively activated receptor (CAR). J Biol Chem. 2004;279:38379–85.
Glaeser H, Drescher S, Eichelbaum M, Fromm MF. Influence of rifampicin on the expression and function of human intestinal cytochrome P450 enzymes. Br J Clin Pharmacol. 2005;59:199–206.
Rane Y, Mashru R, Sankalia M, Sankalia J. Effect of hydrophilic swellable polymers on dissolution enhancement of carbamazepine solid dispersions studied using response surface methodology. AAPS PharmSciTech. 8:Article 27. 2007.
Rege BD, Kao JP, Polli JE. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharm Sci. 2002;16:237–46.
Rege BD, Yu LX, Hussain AS, Polli JE. Effect of common excipients on Caco-2 transport of low-permeability drugs. J Pharm Sci. 2001;90:1776–86.
Aitken AE, Morgan ET. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos. 2007;35:1687–93.
Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, et al. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006;281:17882–9.
Aitken AE, Lee CM, Morgan ET. Roles of nitric oxide in inflammatory downregulation of human cytochromes P450. Free Radic Biol Med. 2008;44:1161–8.
Pondugula SR, Dong H, Chen T. Phosphorylation and protein-protein interactions in PXR-mediated CYP3A repression. Expert Opin Drug Metab Toxicol. 2009;5:861–73.
ACKNOWLEDGEMENTS
The authors thank Dr. Steve Kliewer (University of Texas, Southwestern Medical Center, Dallas, TX) for providing the pSG5-hPXR expression vector. Human liver tissues were procured with the assistance of Jennifer Fuhrman from the University of Maryland Medical Center (Baltimore, MD). This research was partly supported by National Institute of Health Grant (R01, DK061652).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tompkins, L., Lynch, C., Haidar, S. et al. Effects of Commonly Used Excipients on the Expression of CYP3A4 in Colon and Liver Cells. Pharm Res 27, 1703–1712 (2010). https://doi.org/10.1007/s11095-010-0170-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0170-2