ABSTRACT
Purpose
In vivo efficacy and safety of HPMA-based copolymers armed with doxorubicin via a spacer containing pH-sensitive linkage that can be prepared within a broad range of attached drug contents (1) was tested in murine tumor models.
Methods
Mice bearing T cell lymphoma EL4 or B cell lymphoma 38C13 were treated with a single dose of the conjugate (15, 25, and 75 mg Dox eq./kg i.v.) in a therapeutic regime. Anti-tumor resistance of the cured animals was proved by a second challenge with a lethal dose of tumor cells without additional treatment.
Results
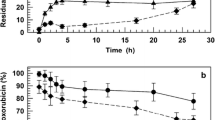
The content of drug bound to the polymer is an important parameter in relation to the conjugate therapeutic efficacy. The best anti-tumor effects were produced by conjugates with 10 – 13 wt% of bound doxorubicin. Free doxorubicin up to 4.6% relative to total drug content had no impact on the treatment efficacy and acute toxicity. The conjugates induced a complete cure of mice and regular treatment-dependent development of specific anti-tumor resistance. No myelosuppression or organ damage was observed.
Conclusions
A well-defined HPMA copolymer-doxorubicin conjugate with pH-sensitive drug release is a good candidate for clinical trials as it has remarkable anti-tumor efficacy and a favorable safety profile.






Similar content being viewed by others
Abbreviations
- B/6:
-
C57BL/6
- CRT:
-
calreticulin
- Dox:
-
doxorubicin
- HMGB1:
-
high mobility group box-1
- HPMA:
-
N-(2-hydroxypropyl)methacrylamide
- PBS:
-
phosphate buffered saline
REFERENCES
Etrych T, Mrkvan T, Chytil P, Konak C, Rihova B, Ulbrich K. N-(2-Hydroxypropyl)methacrylamide-based polymer conjugates with pH-controlled activation of doxorubicin. I. New synthesis, physicochemical characterization and preliminary biological evaluation. J Appl Polym Sci. 2008;109:3050–61.
Ulbrich K, Etrych T, Chytil P, Pechar M, Jelinkova M, Rihova B. Polymeric anticancer drugs with pH-controlled activation. Int J Pharm. 2004;277:63–72.
Kopecek J, Kopeckova P, Minko T, Lu ZR, Peterson CM. Water soluble polymers in tumor targeted delivery. J Control Release. 2001;74:147–58.
Seymour LW, Ferry DR, Anderson D, Hesslewood S, Julyan PJ, Poyner R, et al. Hepatic drug targeting: phase I evaluation of polymer-bound doxorubicin. J Clin Oncol. 2002;20:1668–76.
Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6:688–701.
Rihova B. Immunomodulating activities of soluble synthetic polymer-bound drugs. Adv Drug Deliv Rev. 2002;54:653–74.
Rihova B, Kovar L, Kovar M, Hovorka O. Cytotoxicity and immunostimulation: double attack on cancer cells with polymeric therapeutics. Trends Biotechnol. 2009;27:11–7.
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92.
Duncan R, Cable HC, Lloyd JB, Rejmanova P, Kopecek J. Polymers containing enzymatically degradable bonds 7. Design of oligopeptide side chains in poly[N-(2-hydroxypropyl)methacrylamide] copolymers to promote efficient degradation by lysosomal enzymes. Makromol Chem. 1984;184:1997–2008.
Kratz F, Beyer U, Schutte MT. Drug-polymer conjugates containing acid-cleavable bonds. Crit Rev Ther Drug Carrier Syst. 1999;16:245–88.
Etrych T, Jelinkova M, Rihova B, Ulbrich K. New HPMA copolymers containing doxorubicin bound via pH-sensitive linkage: synthesis and preliminary in vitro and in vivo biological properties. J Control Release. 2001;73:89–102.
Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–84.
Etrych T, Chytil P, Jelinkova M, Rihova B, Ulbrich K. Synthesis of HPMA copolymers containing doxorubicin bound via a hydrazone linkage. Effect of spacer on drug release and in vitro cytotoxicity. Macromolecular Bioscience. 2002;2:43–52.
Kovar M, Kovar L, Subr V, Etrych T, Ulbrich K, Mrkvan T, et al.. HPMA copolymers containing doxorubicin bound by a proteolytically or hydrolytically cleavable bond: comparison of biological properties in vitro. J Control Release. 2004;99:301–14.
Kovar L, Strohalm J, Chytil P, Mrkvan T, Kovar M, Hovorka O, et al. The same drug but a different mechanism of action: comparison of free doxorubicin with two different N-(2-hydroxypropyl)methacrylamide copolymer-bound doxorubicin conjugates in EL-4 cancer cell line. Bioconjug Chem. 2007;18:894–902.
Hovorka O, Etrych T, Subr V, Strohalm J, Ulbrich K, Rihova B. HPMA based macromolecular therapeutics: internalization, intracellular pathway and cell death depend on the character of covalent bond between the drug and the peptidic spacer and also on spacer composition. J Drug Target. 2006;14:391–403.
Rihova B, Etrych T, Pechar M, Jelinkova M, Stastny M, Hovorka O, et al. Doxorubicin bound to a HPMA copolymer carrier through hydrazone bond is effective also in a cancer cell line with a limited content of lysosomes. J Control Release. 2001;74:225–32.
Etrych T, Chytil P, Mrkvan T, Sirova M, Rihova B, Ulbrich K. Conjugates of doxorubicin with graft HPMA copolymers for passive tumor targeting. J Control Release. 2008;132:184–92.
Putnam DA, Shiah JG, Kopecek J. Intracellularly biorecognizable derivatives of 5-fluorouracil. Implications for site-specific delivery in the human condition. Biochem Pharmacol. 1996;52:957–62.
Ulbrich K, Subr V, Strohalm J, Plocova D, Jelinkova M, Rihova B. Polymeric drugs based on conjugates of synthetic and natural macromolecules I. Synthesis and physico-chemical characterisation. J Control Release. 2000;64:63–79.
Ulbrich K, Etrych T, Chytil P, Jelinkova M, Rihova B. Antibody-targeted polymer-doxorubicin conjugates with pH-controlled activation. J Drug Target. 2004;12:477–89.
Bergman Y, Haimovich J. Characterization of a carcinogen-induced murine B lymphocyte cell line of C3H/eB origin. Eur J Immunol. 1977;7:413–7.
Mrkvan T, Sirova M, Etrych T, Chytil P, Strohalm J, Plocova D, et al. Chemotherapy based on HPMA copolymer conjugates with pH-controlled release of doxorubicin triggers anti-tumor immunity. J Control Release. 2005;110:119–29.
Shen WC, Ryser HJ. cis-Aconityl spacer between daunomycin and macromolecular carriers: a model of pH-sensitive linkage releasing drug from a lysosomotropic conjugate. Biochem Biophys Res Commun. 1981;102:1048–54.
Choi W-M, Kopeckova P, Minko T, Kopecek J. Synthesis of HPMA copolymer containing adriamycin bound via an acid-labile spacer and its activity toward human ovarian carcinoma cells. J Bioact Comp Polym. 1999;14:447–56.
Kaneko T, Willner D, Monkovic I, Knipe JO, Braslawsky GR, Greenfield RS, et al. New hydrazone derivatives of adriamycin and their immunoconjugates-a correlation between acid stability and cytotoxicity. Bioconjug Chem. 1991;2:133–41.
Ulbrich K, Etrych T, Chytil P, Jelinkova M, Rihova B. HPMA copolymers with pH-controlled release of doxorubicin: in vitro cytotoxicity and in vivo antitumor activity. J Control Release. 2003;87:33–47.
Duncan R, Cable HC, Rejmanova P, Kopecek J, Lloyd JB. Tyrosinamide residues enhance pinocytic capture of N-(2-hydroxypropyl)methacrylamide copolymers. Biochim Biophys Acta. 1984;799:1–8.
Ulbrich K, Konák C, Tuzar Z, Kopecek J. Solution properties of drug carriers based on poly[N-(2-hydroxypropyl)methacrylamide] containing biodegradable bonds. Makromol Chem. 1987;188:1261–72.
Seymour LW, Duncan R, Strohalm J, Kopecek J. Effect of molecular weight (Mw) of N-(2-hydroxypropyl)methacrylamide copolymers on body distribution and rate of excretion after subcutaneous, intraperitoneal, and intravenous administration to rats. J Biomed Mater Res. 1987;21:1341–58.
Maccubbin DL, Mace KF, Ehrke MJ, Mihich E. Modification of host antitumor defense mechanisms in mice by progressively growing tumor. Cancer Res. 1989;49:4216–24.
Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–5.
Zhou S, Starkov A, Froberg MK, Leino RL, Wallace KB. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Cancer Res. 2001;61:771–7.
Rihova B, Kopeckova P, Strohalm J, Rossmann P, Vetvicka V, Kopecek J. Antibody-directed affinity therapy applied to the immune system: in vivo effectiveness and limited toxicity of daunomycin conjugated to HPMA copolymers and targeting antibody. Clin Immunol Immunopathol. 1988;46:100–14.
Rihova B, Bilej M, Vetvicka V, Ulbrich K, Strohalm J, Kopecek J, et al. Biocompatibility of N-(2-hydroxypropyl) methacrylamide copolymers containing adriamycin. Immunogenicity, and effect on haematopoietic stem cells in bone marrow in vivo and mouse splenocytes and human peripheral blood lymphocytes in vitro. Biomaterials. 1989;10:335–42.
Minko T, Kopeckova P, Kopecek J. Efficacy of the chemotherapeutic action of HPMA copolymer-bound doxorubicin in a solid tumor model of ovarian carcinoma. Int J Cancer. 2000;86:108–17.
Gianasi E, Wasil M, Evagorou EG, Keddle A, Wilson G, Duncan R. HPMA copolymer platinates as novel antitumour agents: in vitro properties, pharmacokinetics and antitumour activity in vivo. Eur J Cancer. 1999;35:994–1002.
Duncan R, Seymour LW, O’Hare KB, Flanagan PA, Wedge S, Hume IC, et al. Preclinical evaluation of polymer-bound doxorubicin. J Control Rel. 1992;19:331–46.
Rihova B, Strohalm J, Hoste K, Jelínkova M, Hovorka O, Kovar M, et al. Immunoprotective therapy with targeted anticancer drugs. Macromol Symp. 2001;172:21–8.
Yeung TK, Hopewell JW, Simmonds RH, Seymour LW, Duncan R, Bellini O, et al. Reduced cardiotoxicity of doxorubicin given in the form of N-(2-hydroxypropyl)methacrylamide conjugates: and experimental study in the rat. Cancer Chemother Pharmacol. 1991;29:105–11.
Hopewel JW, Duncan R, Wilding D, Chakrabarti K. Preclinical evaluation of the cardiotoxicity of PK2: a novel HPMA copolymer-doxorubicin-galactosamine conjugate antitumour agent. Hum Exp Toxicol. 2001;20:461–70.
Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R, et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates Cancer Research Campaign Phase I/II Committee. Clin Cancer Res. 1999;5:83–94.
Rihova B, Strohalm J, Prausova J, Kubackova K, Jelinkova M, Rozprimova L, et al. Cytostatic and immunomobilizing activities of polymer-bound drugs: experimental and first clinical data. J Control Release. 2003;91:1–16.
Rihova B, Strohalm J, Kubackova K, Jelinkova M, Rozprimova L, Sirova M, et al. Drug-HPMA-HuIg conjugates effective against human solid cancer. Adv Exp Med Biol. 2003;519:125–43.
Rihova B, Strohalm J, Kubackova K, Jelinkova M, Hovorka O, Kovar M, et al. Acquired and specific immunological mechanisms co-responsible for efficacy of polymer-bound drugs. J Control Release. 2002;78:97–114.
Sirova M, Strohalm J, Subr V, Plocova D, Rossmann P, Mrkvan T, et al. Treatment with HPMA copolymer-based doxorubicin conjugate containing human immunoglobulin induces long-lasting systemic anti-tumour immunity in mice. Cancer Immunol Immunother. 2007;56:35–47.
Rihova B, Strohalm J, Kovar M, Mrkvan T, Subr V, Hovorka O, et al. Induction of systemic antitumor resistance with targeted polymers. Scand J Immunol. 2005;62:100–5.
Kovar M, Tomala J, Chmelova H, Kovar L, Mrkvan T, Joskova R, et al. Overcoming immunoescape mechanisms of BCL1 leukemia and induction of CD8+ T-cell-mediated BCL1-specific resistance in mice cured by targeted polymer-bound doxorubicin. Cancer Res. 2008;68:9875–83.
Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9.
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61.
Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, et al. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol. 2008;20:504–11.
ACKNOWLEDGEMENTS
The work was supported by grant KAN 200200651, Premium Academiae, and Institutional Research Concept AV0Z50200510. Authors appreciate the funding support from pharmaceutical company Zentiva, k.s. (Czech Republic), and thank Mrs. Helena Mišurcová and Ms. Pavlína Jungrová for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sirova, M., Mrkvan, T., Etrych, T. et al. Preclinical Evaluation of Linear HPMA-Doxorubicin Conjugates with pH-Sensitive Drug Release: Efficacy, Safety, and Immunomodulating Activity in Murine Model. Pharm Res 27, 200–208 (2010). https://doi.org/10.1007/s11095-009-9999-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9999-7