Abstract
Methods
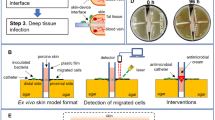
In this study we determined, for the first time, the ability of microorganisms to traverse microneedle-induced holes using two different in vitro models.
Results
When employing Silescol® membranes, the numbers of Candida albicans, Pseudomonas aeruginosa and Staphylococcus epidermidis crossing the membranes were an order of magnitude lower when the membranes were punctured by microneedles rather than a 21G hypodermic needle. Apart from the movement of C. albicans across hypodermic needle-punctured membranes, where 40.2% of the microbial load on control membranes permeated the barrier over 24 h, the numbers of permeating microorganisms was less than 5% of the original microbial load on control membranes. Experiments employing excised porcine skin and radiolabelled microorganisms showed that the numbers of microorganisms penetrating skin beyond the stratum corneum were approximately an order of magnitude greater than the numbers crossing Silescol® membranes in the corresponding experiments. Approximately 103 cfu of each microorganism adhered to hypodermic needles during insertion. The numbers of microorganisms adhering to MN arrays were an order of magnitude higher in each case.
Conclusion
We have shown here that microneedle puncture resulted in significantly less microbial penetration than did hypodermic needle puncture and that no microorganisms crossed the viable epidermis in microneedle—punctured skin, in contrast to needle-punctured skin. Given the antimicrobial properties of skin, it is, therefore, likely that application of microneedle arrays to skin in an appropriate manner would not cause either local or systemic infection in normal circumstances in immune-competent patients. In supporting widespread clinical use of microneedle-based delivery systems, appropriate animal studies are now needed to conclusively demonstrate this in vivo. Safety in patients will be enhanced by aseptic or sterile manufacture and by fabricating microneedles from self-disabling materials (e.g. dissolving or biodegradable polymers) to prevent inappropriate or accidental reuse.






Similar content being viewed by others
References
Prausniz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56:581–7.
Mikszta JA, Dekker JP, Harvey NG, Dean CH, Brittingham JM. Microneedle-based intradermal delivery of the anthrax recombinant protective antigen vaccine. Infect Immun. 2006;74:6806–10.
Coulman SA, Barrow D, Anstey A, Gateley C, Morrissey A, Wilke N. Minimally invasive cutaneous delivery of macromolecules and plasmid DNA via microneedles. Curr Drug Deliv. 2006;3:65–75.
Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci. 1998;87:922–5.
Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Cont Rel. 2005;104:51–66.
Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Cont Rel. 2004;97:503–11.
Wang PM, Cornwell M, Hill J, Prausnitz MR. Precise microinjection into skin using hollow microneedles. J Invest Dermatol. 2006;126:1080–7.
McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci USA. 2003;100:13755–60.
Teo MA, Shearwood C, Ng KC, Lu J, Moochhala S. In vitro and in vivo characterization of MEMS microneedles. Biomed Microdev. 2005;7:47–52.
Donnelly RF, Morrow DIJ, McCarron PA, Woolfson AD, Morrissey A, Juzenas P, et al. Microneedle-mediated intradermal delivery of 5-aminolevulinic acid: potential for enhanced topical photodynamic therapy. J Cont Rel. 2008;129:154–62.
Donnelly RF, Morrow DIJ, McCarron PA, Woolfson AD, Morrissey A, Juzenas P, et al. Microneedle arrays permit true intradermal delivery of a preformed photosensitiser. Photochem Photobiol. 2009;85:195–204.
Woolfson AD, McCafferty DF, McCallion CR, McAdams ET, Anderson JMcC. Moisture-activated, electrically conducting bioadhesive hydrogels as interfaces for bioelectrodes: effect of film hydration on cutaneous adherence in wet environments. J Appl Polym Sci. 1995;58:1291–6.
Fourtanier A, Berrebi C. Miniature pig as an animal model to study photoaging. Photochem Photobiol. 1989;50:771–84.
Moser K, Kriwet K, Froehlich C, Kalia YN, Guy RH. Supersaturation: enhancement of skin penetration and permeation of a lipophilic drug. Pharm Res. 2001;18:1006–11.
Pellett MA, Roberts MS, Hadgraft J. Supersaturated solutions evaluated with an in vitro stratum corneum tape stripping technique. Int J Pharm. 1997;151:91–8.
Wermeling DP, Banks SL, Huclson DA, Prausnitz M. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Nat Acad Sci USA. 2008;105:2058–63.
Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdev. 2009;11:35–47.
Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–9.
Alarcon JB, Hartley AW, Harvey NG, Mikszta JA. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin Vacc Immun. 2007;14:375–81.
Seal DV, Hay RJ, Middleton KR. Skin and wound infection: investigation and treatment in practice. London: Informa Healthcare; 2000. p. 5–8.
www.who.int/en. Accessed 16th April 2009.
Pittet D, Allegranzi B, Sax H, Dharan S, Pessoa-Silva CL, Donaldson L, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006;6:641–52.
Del Mar CB, Glasziou PP, Spinks AB, Sanders SL. Is isopropyl alcohol swabbing before injection really necessary? Med J Aust. 2001;175:341–2.
Elsner P. Antimicrobials and the skin physiological and pathological flora. Curr Prob Derm. 2006;33:35–41.
Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24:1653–64.
Acknowledgements
This work was supported by BBSRC grant number: BB/E020534/1 and the Science Foundation Ireland Tyndall National Access Programme project number NAP 156.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Donnelly, R.F., Singh, T.R.R., Tunney, M.M. et al. Microneedle Arrays Allow Lower Microbial Penetration Than Hypodermic Needles In Vitro . Pharm Res 26, 2513–2522 (2009). https://doi.org/10.1007/s11095-009-9967-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9967-2