Abstract
Purpose
In transdermal drug delivery system (TDDS), chemical enhancers and crystallization inhibitors added into the adhesive matrixes to improve drug permeation and formulation stability often result in some negative effect on adhesive properties and dressing performance. The aim of this paper is to develop a hydrophilic pressure sensitive adhesive (PSA) for TDDS without using additional chemical enhancers and crystallization inhibitors.
Methods
A quaternary blend (PDGW) composed of polyvinyl pyrrolidone, D,L-lactic acid oligomers, glycerol and water was prepared. The adhesive strength, drug loading capacity, drug state and stability of PDGW were characterized by using ibuprofen (IBU) and salicylic acid (SA) as model drugs. Moreover, In vitro and in vivo drug permeation through rat skin from PDGW patch in comparison to acrylate adhesive (ACA) and nature rubber adhesive (NRA) was investigated.
Results
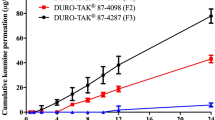
PDGW performs excellent drug loading and crystallization inhibition capacity. Furthermore, the accumulative amount for 24 h in vitro from PDGW patch is far higher than that from ACA and NRA patch. And the plasma concentration of drugs in vivo from PDGW patch is bigger than that from ACA patch.
Conclusions
PDGW possesses excellent PSA properties and self-enhancement for drug percutaneous permeation, which can be used to develop new formulation of TDDS.






Similar content being viewed by others
References
A. M. Wokovich, S. Prodduturi, W. H. Doub, A. S. Hussain, and L. F. Buhse. Transdermal drug delivery system (TDDS) adhesion as a critical safety, efficacy and quality attribute. Eur. J. Pharm. Biopharm. 64:1–8 (2006). doi:10.1016/j.ejpb.2006.03.009.
C. Valenta, and B.G. Auner. The use of polymers for dermal and transdermal delivery. Eur. J. Pharm. Biopharm. 58:279–289 (2004). doi:10.1016/j.ejpb.2004.02.017.
S. Venkatraman, and R. Gale. Skin adhesives and skin adhesion: 1. Transdermal drug delivery systems. Biomaterials. 19:1119–1136 (1998). doi:10.1016/S0142-9612(98)00020-9.
H. S. Tan, and W.R. Pfister. Pressure-sensitive adhesives for transdermal drug delivery systems. PSTT. 2:60–69 (1999).
S. Mitragotri. Synergistic effect of enhancers for transdermal drug delivery. Pharm. Res. 17:1354–1359 (2000). doi:10.1023/A:1007522114438.
A. C. Williams, and B. W. Barry. Penetration enhancers. Adv. Drug. Deli. Rev. 56:603–618 (2004). doi:10.1016/j.addr.2003.10.025.
S. M. Taghizadeh, and F. Lahootifard. Effect of different skin permeation enhancers on peel strength of an acrylic PSA. J. Appl. Polym. Sci. 90:2987–2991 (2003). doi:10.1002/app.12931.
S. R. Trenor, A.E. Suggs, and B.J. Love. Influence of penetration enhancers on the thermomechanical properties and peel strength of a poly(isobutylene) pressure sensitive adhesive. J. Mater. Sci. Lett. 21:1321–1323 (2002). doi:10.1023/A:1019748112291.
P. N. Kotiyan, and P.R. Vavia. Eudragits: role as crystallization inhibitors in drug in adhesive transdermal systems of estradiol. Eur. J. Pharm. Biopharm. 52:173–180 (2001). doi:10.1016/S0939-6411(01)00174-6.
F. Cilurzo, P. Minghetti, A. Casiraghi, L. Tosi, S. Pagani, and L. Montanari. Polymethacrylates as crystallization inhibitors in monolayer transdermal patches containing Ibuprofen. Eur. J. Pharm. Biopharm. 60:61–66 (2005). doi:10.1016/j.ejpb.2005.02.001.
M. M. Feldstein, V. N. Tohmakhchi, L.B. Malkhazov, A.E. Vasiliev, and N.A. Platé. Hydrophilic polymeric matrixes for enhanced transdermal drug delivery. Int. J. Pharm. 131:229–242 (1996). doi:10.1016/0378-5173(95)04351-9.
T. Loftsson, and A.M. Sigurðardóttir. The effect of polyvinylpyrrolidone and hydroxypropyl methylcellulose on HPβCD complexation of hydrocortisone and its permeability through hairless mouse skin. Eur. J. Pharm. Sci. 2:297–301 (1994). doi:10.1016/0928-0987(94)90013-2.
B. Mukherjee, S. Mahapatra, R. Gupta, B. Patra, A. Tiwari, and P. Arora. A comparison between povidone–ethylcellulose and povidone–eudragit transdermal dexamethasone matrix patches based on in vitro skin permeation. Eur. J. Pharm. Biopharm. 59:475–483 (2005). doi:10.1016/j.ejpb.2004.09.009.
J. H. Zhang, L. D. Deng, H. J. Zhao, M. Liu, H. J. Jin, J. Q. Li, and A. J. Dong. Pressure sensitive adhesive properties of poly(N-vinyl pyrrolidone)/D,L-lactic acid oligomer/glycerol/water blends for TDDS. J. Biomater. Sci. Polym. Ed (2009). doi:10.1163/156856209X410111.
N. Wang, and X. S. Wu. Synthesis, characterization, biodegradation and drug delivery application of biodegradable lactic/glycolic acid oligomers: Part II. Biodegradation and drug delivery application. J. Biomater. Sci. Polym. Ed. 9:75–87 (1997). doi:10.1163/156856297X00272.
M. Aqil, and A. Ali. Monolithic matrix type transdermal drug delivery systems of pinacidil monohydrate: in vitro characterization. Eur. J. Pharm. Biopharm. 54:161–164 (2002). doi:10.1016/S0939-6411(02)00059-0.
W. H. Gardner. Water content. In A. Kline (ed.), Methods of Soil Analysis, 2nd ed. American Society of Agronomy, Madison, WI, 1986, pp. 493–544.
A. Gal, and A. Nussinovitch. Plasticizers in the manufacture of novel skin-bioadhesive patches. Int. J. Pharm. doi:10.1016/j.ijpharm. 2008.11.015 (2008).
M. J. Maurice. The determination of carboxyl groups in polycaprolactam. Anal. Chim. Acta. 26:406–409 (1962). doi:10.1016/S0003-2670(00)88406-8.
S. M. Al-Saidan. Transdermal self-permeation enhancement of ibuprofen. J. Control. Release. 100:199–209 (2004). doi:10.1016/j.jconrel.2004.08.011.
N. H. Gabboun, N.M. Najib, H.G. Ibrahim, and S. Assaf. Release of salicylic acid, diclofenac acid and diclofenac acid salts from isotropic and anisotropic nonionic surfactant systems across rat skin. Int. J. Pharm. 212:73–80 (2001). doi:10.1016/S0378-5173(00)00585-8.
L. Simonsen, A. Jørgensen, E. Benfeldt, and L. Groth. Differentiated in vivo skin penetration of salicylic compounds in hairless rats measured by cutaneous microdialysis. Eur. J. Pharm Sci. 21:379–388 (2004). doi:10.1016/j.ejps.2003.11.004.
J. H. Kim, and H.K. Choi. Effect of additives on the crystallization and the permeation of ketoprofen from adhesive matrix. Int. J. Pharm. 236:81–85 (2002). doi:10.1016/s0378-5173(02)00017-0.
S. Y. Lin, C.J. Lee, and Y.Y. Lin. Drug–polymer interaction affecting the mechanical properties, adhesion strength and release kinetics of piroxicam-loaded Eudragit E films plasticized with different plasticizers. J. Control. Release. 33:375–381 (1995). doi:10.1016/0168-3659(94)00109-8.
D. G. Maillard-Salin, Ph. Bécourt, and G. Couarraze. A study of the adhesive–skin interface: correlation between adhesion and passage of a drug. Int. J. Pharm. 200:121–126 (2000). doi:10.1016/S0378-5173(00)00369-0.
T. Kokubo, K. Sugibayashi, and Y. Morimoto. Interaction between drugs and pressure-sensitive adhesives in transdermal therapeutic systems. Pharm. Res. 11:104–107 (1994). doi:10.1023/A:1018906013527.
K. Y. Ho, and K. Dodou. Rheological studies on pressure-sensitive silicone adhesives and drug-in-adhesive layers as a means to characterise adhesive performance. Int. J. Pharm. 333:24–33 (2007). doi:10.1016/j.ijpharm.2006.09.043.
J. Hirvonen, J. H. Rytting, P. Paronen, and A. Urtti. Dodecyl N,N-dimethylamino acetate and azone enhance drug penetration across human, snake, and rabbit skin. Pharm. Res. 8:933–936 (1991). doi:10.1023/A:1015824100788.
X. H. Ma, J. Taw, and C.M. Chinan. Control of drug crystallization in transdermal matrix system. Int. J. Pharm. 142:115–119 (1996). doi:10.1016/0378-5173(96)04647-9.
K. H. Ziller, and H. Rupprecht. Control of crystal growth in drug suspensions. Drug Dev. Ind. Pharm. 14:2341–2370 (1988). doi:10.3109/03639048809152019.
L. S. Taylor, and G. Zografi. Spectroscopic characterization of interaction between PVP and indomethacin in amorphous molecular dispersions. Pharm. Res. 14:1691–1697 (1997). doi:10.1023/A:1012167410376.
M. Yoshioka, B.C. Hancock, and G. Zografi. Inhibition of indomethacin crystallization in poly(vinylpyrrolidone) coprecipitates. J. Pharm. Sci. 84:983–986 (1995). doi:10.1002/jps.2600840814.
N. M. Najib, M. Suleiman, and A. Malakh. Characteristics of the in vitro release of ibuprofen from polyvinylpyrrolidone solid dispersions. Int. J. Pharm. 32:229–236 (1986). doi:10.1016/0378-5173(86)90183-3.
B. Yuan, C. McGlinchey, and E. M. Pearce. Explanation of tackifier effect on the viscoelastic properties of polyolefin-based pressure sensitive adhesives. J. Appl. Polym. Sci. 99:2408–2413 (2006). doi:10.1002/app.22820.
M. B. Novikov, A. Roos, C. Creton, and M. M. Feldstein. Dynamic mechanical and tensile properties of poly(N-vinyl pyrrolidone)–poly(ethylene glycol) blends. Polymer. 44:3561–3578 (2003). doi:10.1016/S0032-3861(03)00132-0.
P. R. Rao, M. N. Reddy, S. Ramakrishna, and P. V. Diwan. Comparative in vivo evaluation of propranolol hydrochloride after oral and transdermal administration in rabbits. Eur. J. Pharm. Biopharm. 56:81–85 (2003). doi:10.1016/S0939-6411(03)00038-9.
J. M. Barichello, N. Yamakawa, M. Kisyuku, H. Handa, T. Shibata, T. Ishida, and H. Kiwada. Combined effect of liposomalization and addition of glycerol on the transdermal delivery of isosorbide 5-nitrate in rat skin. Int. J. Pharm. 357:199–205 (2008). doi:10.1016/j.ijpharm.2008.01.052.
M. Hara, and A.S. Verkman. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 100:7360–7365 (2003). doi:10.1073/pnas.1230416100.
O. Diez-Sales, A. C. Watkinson, M. Herráez-Dominguez, C. Javaloyes, and J. Hadgraft. A mechanistic investigation of the in vitro human skin permeation enhancing effect of Azone. Int. J. Pharm. 129:33–40 (1996). doi:10.1016/0378-5173(95)04237-7.
S. C. Chi, E. S. Park, and H. Kim. Effect of penetration enhancers on flurbiprofen permeation through rat skin. Int. J. Pharm. 126:267–274 (1995). doi:10.1016/0378-5173(95)04137-0.
J. Y. Fang, T. L. Hwang, C. L. Fang, and H. C. Chiu. In vitro and in vivo evaluations of the efficacy and safety of skin permeation enhancers using flurbiprofen as a model drug. Int. J. Pharm. 255:153–166 (2003). doi:10.1016/S0378-5173(03)00086-3.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (Number 30672554).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 72.0 kb)
Rights and permissions
About this article
Cite this article
Zhang, J., Liu, Z., Du, H. et al. A Novel Hydrophilic Adhesive Matrix with Self-Enhancement for Drug Percutaneous Permeation Through Rat Skin. Pharm Res 26, 1398–1406 (2009). https://doi.org/10.1007/s11095-009-9850-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9850-1