Abstract
Purpose
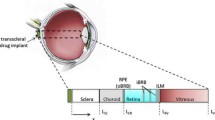
The direct penetration route following transscleral drug administration presents several barrier and clearance mechanisms—including loss to choroidal blood flow, active transport by the retinal pigment epithelium (RPE), and loss to the conjunctival lymphatics and episcleral blood vessels. The objective of this research was to quantify the role of choroidal and episcleral losses.
Materials and Methods
A finite element model was created for drug distribution in the posterior human eye. The volumetric choroidal loss constant, active transport component and mass transfer from the scleral surface were unknown parameters in the model. The model was used to simulate drug distribution from a systemic source, and the results were compared to existing experimental results to obtain values for the parameters.
Results
The volumetric choroidal loss constant, mass transfer coefficient from the scleral surface and active transport component were evaluated to be (2.0 ± 0.6) × 10−5 s−1, (2.0 ± 0.35) × 10−5 cm/s and 8.54 × 10−6 cm/s respectively.
Conclusion
Loss to the choroidal circulation was small compared to loss from the scleral surface. Active transport was predicted to induce periscleral movement of the drug, resulting in more rapid distribution and elevated drug concentrations in the choroid and sclera.






Similar content being viewed by others
References
T. Yasukawa, Y. Ogura, Y. Tabata, H. Kimura, P. Wiedemann, and Y. Honda. Drug delivery systems for vitreoretinal diseases. Prog. Retin. Eye Res. 23:253–281 (2004). doi:10.1016/j.preteyeres.2004.02.003.
G. M. Bleeker, N. J. van Haeringen, E. R. Maas, and E. Glasius. Selective properties of the vitreous barrier. Exp. Eye Res. 7:37–46 (1968). doi:10.1016/S0014-4835(68)80024-7.
P. M. Hughes, O. Olejnik, J. E. Chang-Lin, and C. G. Wilson. Topical and systemic drug delivery to the posterior segments. Adv. Drug Deliv. Rev. 57:2010–2032 (2005). doi:10.1016/j.addr.2005.09.004.
D. H. Geroski, and H. F. Edelhauser. Drug delivery for posterior segment eye disease. Invest. Ophthalmol. Vis. Sci. 41:961–964 (2000).
I. Ahmed, and T. F. Patton. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest. Ophthalmol. Vis. Sci. 26:584–587 (1985).
J. Ambati, E. S. Gragoudas, J. W. Miller, T. T. You, K. Miyamoto, F. C. Delori, and A. P. Adamis. Transscleral delivery of bioactive protein to the choroid and retina. Invest. Ophthalmol. Vis. Sci. 41:1186–1191 (2000).
J. Ambati, C. S. Canakis, J. W. Miller, E. S. Gragoudas, A. Edwards, D. J. Weissgold, I. Kim, F. C. Delori, and A. P. Adamis. Diffusion of high molecular weight compounds through sclera. Invest. Ophthalmol. Vis. Sci. 41:1181–1185 (2000).
A. Bill. Movement of albumin and dextran through the sclera. Arch. Ophthalmol. 74:248–252 (1965).
D. M. Maurice, and J. Polgar. Diffusion across the sclera. Exp. Eye Res. 25:577–582 (1977). doi:10.1016/0014-4835(77)90136-1.
T. W. Olsen, H. F. Edelhauser, J. I. Lim, and D. H. Geroski. Human scleral permeability. Effects of age, cryotherapy, transscleral diode laser, and surgical thinning. Invest. Ophthalmol. Vis. Sci. 36:1893–1903 (1995).
S. C. Pflugfelder, E. Hernandez, S. J. Fliesler, J. Alvarez, M. E. Pflugfelder, and R. K. Forster. Intravitreal vancomycin. Retinal toxicity, clearance, and interaction with gentamicin. Arch. Ophthalmol. 105:831–837 (1987).
S. Tsuboi. Measurement of the volume flow and hydraulic conductivity across the isolated dog retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 28:1776–1782 (1987).
J. G. Cunha-Vaz, and D. M. Maurice. The active transport of fluorescein by the retinal vessels and the retina. J. Physiol. 191:467–486 (1967).
M. Kimura, M. Araie, and S. Koyano. Movement of carboxyfluorescein across retinal pigment epithelium-choroid. Exp. Eye Res. 63:51–56 (1996). doi:10.1006/exer.1996.0090.
S. Koyano, M. Araie, and S. Eguchi. Movement of fluorescein and its glucuronide across retinal pigment epithelium-choroid. Invest. Ophthalmol. Vis. Sci. 34:531–538 (1993).
A. Yoshida, S. Ishiko, and M. Kojima. Outward permeability of the blood-retinal barrier. Graefes Arch. Clin. Exp. Ophthalmol. 230:78–83 (1992). doi:10.1007/BF00166767.
M. S. Stay, J. Xu, T. W. Randolph, and V. H. Barocas. Computer simulation of convective and diffusive transport of controlled-release drugs in the vitreous humor. Pharm. Res. 20:96–102 (2003). doi:10.1023/A:1022207026982.
M. R. Robinson, S. S. Lee, H. Kim, S. Kim, R. J. Lutz, C. Galban, P. M. Bungay, P. Yuan, N. S. Wang, J. Kim, and K. G. Csaky. A rabbit model for assessing the ocular barriers to the transscleral delivery of triamcinolone acetonide. Exp. Eye Res. 82:479–487 (2006). doi:10.1016/j.exer.2005.08.007.
I. A. Sigal, J. G. Flanagan, and C. R. Ethier. Factors influencing optic nerve head biomechanics. Invest. Ophthalmol. Vis. Sci. 46:4189–4199 (2005). doi:10.1167/iovs.05-0541.
I. Fatt, and B. O. Hedbys. Flow of water in the sclera. Exp Eye Res. 10:243–249 (1970). doi:10.1016/S0014-4835(70)80035-5.
J. Xu, J. J. Heys, V. H. Barocas, and T. W. Randolph. Permeability and diffusion in vitreous humor: implications for drug delivery. Pharm. Res. 17:664–669 (2000). doi:10.1023/A:1007517912927.
V. P. Ranta, and A. Urtti. Transscleral drug delivery to the posterior eye: prospects of pharmacokinetic modeling. Adv. Drug Deliv. Rev. 58:1164–1181 (2006). doi:10.1016/j.addr.2006.07.025.
N. P. Blair, R. C. Zeimer, M. M. Rusin, and J. G. Cunha-Vaz. Outward transport of fluorescein from the vitreous in normal human subjects. Arch. Ophthalmol. 101:1117–1121 (1983).
K. Miyake. Outward transport of fluorescein from vitreous in eyes with intraocular surgeries. Jpn. J. Ophthalmol. 38:1091–1096 (1984).
Y. Oguro, Y. Tsukahara, I. Saito, and T. Kondo. Estimation of the permeability of the blood-retinal barrier in normal individuals. Invest. Ophthalmol. Vis. Sci. 26:969–976 (1985).
A. G. Palestine, and R. F. Brubaker. Pharmacokinetics of fluorescein in the vitreous. Invest. Ophthalmol. Vis. Sci. 21:542–549 (1981).
R. C. Zeimer, N. P. Blair, and J. G. Cunha-Vaz. Pharmacokinetic interpretation of vitreous fluorophotometry. Invest. Ophthalmol. Vis. Sci. 24:1374–1381 (1983).
C. B. Engler, B. Sander, M. Larsen, P. Dalgaard, and H. Lund-Andersen. Fluorescein transport across the human blood-retina barrier in the direction vitreous to blood. Quantitative assessment in vivo. Acta. Ophthalmol. (Copenh). 72:655–662 (1994).
C. R. Ethier, M. Johnson, and J. Ruberti. Ocular biomechanics and biotransport. Annu. Rev. Biomed. Eng. 6:249–273 (2004). doi:10.1146/annurev.bioeng.6.040803.140055.
R. D. Schoenwald, G. S. Deshpande, D. G. Rethwisch, and C. F. Barfknecht. Penetration into the anterior chamber via the conjunctival/scleral pathway. J. Ocular Pharmacol. Ther. 13:41–59 (1997).
R. J. Kaiser, and D. M. Maurice. The diffusion of fluorescein in the lens. Exp. Eye Res. 3:156–165 (1964). doi:10.1016/S0014-4835(64)80030-0.
D. M. Maurice. Drug delivery to the posterior segment from drops. Surv. Ophthalmol. 47(Suppl 1):S41–52 (2002). doi:10.1016/S0039-6257(02)00326-0.
P. Blondeau, J. P. Tetrault, and C. Papamarkakis. Diurnal variation of episcleral venous pressure in healthy patients: a pilot study. J. Glaucoma. 10:18–24 (2001). doi:10.1097/00061198-200102000-00005.
P. J. Missel. Hydraulic Flow and Vascular Clearance Influences on Intravitreal Drug Delivery. Pharm. Res. 19:1636–1647 (2002). doi:10.1023/A:1020940927675.
H. Kim, M. J. Lizak, G. Tansey, K. G. Csaky, M. R. Robinson, P. Yuan, N. S. Wang, and R. J. Lutz. Study of ocular transport of drugs released from an intravitreal implant using magnetic resonance imaging. Ann. Biomed. Eng. 33:150–164 (2005). doi:10.1007/s10439-005-8974-7.
H. Kim, M. R. Robinson, M. J. Lizak, G. Tansey, R. J. Lutz, P. Yuan, N. S. Wang, and K. G. Csaky. Controlled drug release from an ocular implant: an evaluation using dynamic three-dimensional magnetic resonance imaging. Invest. Ophthalmol. Vis. Sci. 45:2722–2731 (2004). doi:10.1167/iovs.04-0091.
N. P. Blair, M. A. Evans, T. S. Lesar, and R. C. Zeimer. Fluorescein and fluorescein glucuronide pharmacokinetics after intravenous injection. Invest. Ophthalmol. Vis. Sci. 27:1107–1114 (1986).
S. Kitano, and S. Nagataki. Transport of fluorescein monoglucuronide out of the vitreous. Invest. Ophthalmol. Vis. Sci. 27:998–1001 (1986).
P. R. Amestoy, and J. Y. L'Excellent. MUMPS Multifrontal massively parallel solver, version 2.0. J. Phys. Condens. Matter. 10:7975 (1998). doi:10.1088/0953-8984/10/36/008.
M. Araie, and D. M. Maurice. The loss of fluorescein, fluorescein glucuronide and fluorescein isothiocyanate dextran from the vitreous by the anterior and retinal pathways. Exp. Eye Res. 52:27–39 (1991). doi:10.1016/0014-4835(91)90125-X.
J. Park, P. M. Bungay, R. J. Lutz, J. J. Augsburger, R. W. Millard, A. Sinha Roy, and R. K. Banerjee. Evaluation of coupled convective-diffusive transport of drugs administered by intravitreal injection and controlled release implant. J. Control Release. 105:279–295 (2005). doi:10.1016/j.jconrel.2005.03.010.
P. Dalgaard, and M. Larsen. Fitting numerical solutions of differential equations to experimental data: a case study and some general remarks. Biometrics. 46:1097–1109 (1990). doi:10.2307/2532451.
J. Larsen, H. Lund-Andersen, and B. Krogsaa. Transient transport across the blood-retina barrier. Bull. Math. Biol. 45:749–758 (1983).
H. Lund-Andersen, B. Krogsaa, M. la Cour, and J. Larsen. Quantitative vitreous fluorophotometry applying a mathematical model of the eye. Invest. Ophthalmol. Vis. Sci. 26:698–710 (1985).
D. L. Vollmer, M. A. Szlek, K. Kolb, L. B. Lloyd, and T. M. Parkinson. In vivo transscleral iontophoresis of amikacin to rabbit eyes. J. Ocul. Pharmacol. Ther. 18:549–558 (2002). doi:10.1089/108076802321021090.
S. P. Ayalasomayajula, and U. B. Kompella. Retinal delivery of celecoxib is several-fold higher following subconjunctival administration compared to systemic administration. Pharm. Res. 21:1797–1804 (2004). doi:10.1023/B:PHAM.0000045231.51924.e8.
T. W. Lee, and J. R. Robinson. Drug delivery to the posterior segment of the eye: some insights on the penetration pathways after subconjunctival injection. J. Ocular Pharmacol Ther. 17:565–572 (2001). doi:10.1089/10807680152729257.
A. Tsuji, I. Tamai, and K. Sasaki. Intraocular penetration kinetics of prednisolone after subconjunctival injection in rabbits. Ophthalmic. Res. 20:31–43 (1988).
N. P. Cheruvu, and U. B. Kompella. Bovine and porcine transscleral solute transport: influence of lipophilicity and the Choroid–Bruch’s layer. Invest. Ophthalmol. Vis. Sci. 47:4513–4522 (2006). doi:10.1167/iovs.06-0404.
S. Tsuboi, and J. E. Pederson. Permeability of the isolated dog retinal pigment epithelium to carboxyfluorescein. Invest. Ophthalmol. Vis. Sci. 27:1767–1770 (1986).
S. Tsuboi, T. Fujimoto, Y. Uchihori, K. Emi, S. Iizuka, K. Kishida, and R. Manabe. Measurement of retinal permeability to sodium fluorescein in vitro. Invest. Ophthalmol. Vis. Sci. 25:1146–1150 (1984).
A. Bill, P. Tornquist, and A. Alm. Permeability of the intraocular blood vessels. Trans. Ophthalmol. Soc. U. K. 100:332–336 (1980).
A. Bill. Capillary permeability to and extravascular dynamics of myoglobin, albumin and gammaglobulin in the uvea. Acta Physiol. Scand. 73:204–219 (1968).
P. Tornquist. Capillary permeability in cat choroid, studied with the single injection technique (II). Acta Physiol. Scand. 106:425–430 (1979).
G. Raviola. Conjunctival and episcleral blood vessels are permeable to blood-borne horseradish peroxidase. Invest. Ophthalmol. Vis. Sci. 24:725–736 (1983).
L. Pitkanen, V. P. Ranta, H. Moilanen, and A. Urtti. Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Invest. Ophthalmol. Vis. Sci. 46:641–646 (2005)doi:10.1167/iovs.04-1051.
H. Lund-Andersen, M. Larsen, P. Dalgaard, and W. Olsen. Fluorescein and fluorescein glucuronide in the vitreous body of diabetic patients. Graefes. Arch. Clin. Exp. Ophthalmol. 225:173–176 (1987). doi:10.1007/BF02175445.
F. Mac Gabhann, A. Demetriades, T. Deering, J. Packer, S. Shah, E. Duh, P. Campochiaro, and A. Popel. Protein transport to choroid and retina following periocular injection: theoretical and experimental study. Ann. Biomed. Eng. 35:615–630 (2007).
R. A. Pontes de Carvalho, M. L. Krausse, A. L. Murphree, E. E. Schmitt, P. A. Campochiaro, and I. H. Maumenee. Delivery from episcleral exoplants. Invest. Ophthalmol. Vis. Sci. 47:4532–4539 (2006). doi:10.1167/iovs.06-0030.
A. Ohtori, and K. Tojo. In vivo/in vitro correlation of intravitreal delivery of drugs with the help of computer simulation. Biol. Pharm. Bull. 17:283–290 (1994).
S. Friedrich, Y. L. Cheng, and B. Saville. Drug distribution in the vitreous humor of the human eye: the effects of intravitreal injection position and volume. Curr. Eye Res. 16:663–669 (1997). doi:10.1076/ceyr.16.7.663.5061.
S. Friedrich, B. Saville, and Y. L. Cheng. Drug distribution in the vitreous humor of the human eye: the effects of aphakia and changes in retinal permeability and vitreous diffusivity. J. Ocular Pharmacol. Ther. 13:445–459 (1997).
S. Friedrich, Y. L. Cheng, and B. Saville. Finite element modeling of drug distribution in the vitreous humor of the rabbit eye. Ann. Biomed. Eng. 25:303–314 (1997). doi:10.1007/BF02648045.
Acknowledgements
This work was supported by the Institute for Engineering and Medicine (IEM) at the University of Minnesota and by the National Institutes of Health (R03-EB007815). The resources provided by the Minnesota Supercomputing Institute (MSI) at the University of Minnesota were used for running the simulations. We thank Phil Bransford, who helped us in the initial stages of model building and Matt Stay for providing valuable insights on the subject matter.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Sensitivity Computation for Gauss–Newton Scheme
Squared curve-fitting problem amounts to minimizing the error between the simulations and experimental results,
where c exp is the average concentration observed experimentally. The Gauss–Newton scheme finds the minimum iteratively, requiring partial derivatives of the objective function E, with respect to the parameters, e.g.,
The sensitivities s γ and s ksc are defined as
The mass balance Eqs. 3, 4 and 5 along with boundary conditions 7, 8 and 9 are differentiated with respect to γ and k sc to obtain the following relationships which would be solved for s γ and s ksc respectively.
Equations and Boundary Conditions for s γ
-
1.
Domain equations
$${\text{Vitreous: }}\frac{{\partial s_\gamma }}{{\partial t}} + v \cdot \nabla s_\gamma - D_v \nabla ^2 s_\gamma = 0$$(14)$${\text{RPE: }}\frac{{\partial s_\gamma }}{{\partial t}} + \left( {v + k_{{\text{act}}} } \right) \cdot \nabla s_\gamma - D_{{\text{rpe}}} \nabla ^2 s_\gamma = - \frac{{\partial k_{{\text{act}}} }}{{\partial \gamma }} \cdot \nabla c$$(15)$${\text{CS: }}\frac{{\partial s_\gamma }}{{\partial t}} + v \cdot \nabla s_\gamma - D_{{\text{cs}}} \nabla ^2 s_\gamma + \gamma s_\gamma = \left( {c_{{\text{bl}}} - c} \right)$$(16) -
2.
Boundary conditions
$${\text{Lens: }}n \cdot \left( { - D\nabla s_\gamma + vs_\gamma } \right) = 0$$(17)$${\text{Hyaloid: }}n \cdot \left( { - D\nabla s_\gamma + vs_\gamma } \right) = k_{{\text{hy}}} s_\gamma $$(18)$${\text{Sclera: }}n \cdot \left( { - D\nabla s_\gamma + vs_\gamma } \right) = k_{{\text{sc}}} s_\gamma $$(19)
where \(\left\| {\frac{{\partial k_{{\text{act}}} }}{{\partial \gamma }}} \right\| = \frac{{{\text{Pe}}^* L_{{\text{rpe}}} }}{{\left( {\frac{{L_{{\text{rpe}}}^2 }}{{D_{{\text{rpe}}} }} + \frac{{L_{{\text{cs}}}^2 }}{{D_{{\text{cs}}} }} + \frac{1}{\gamma }} \right)^2 }}\left( {\frac{1}{{\gamma ^2 }}} \right)\) from Eq. 10, and the vector points in the same direction as kact.
Equations and Boundary Conditions for s ksc
-
1.
Domain equations
$${\text{Vitreous: }}\frac{{\partial s_{{\text{ksc}}} }}{{\partial t}} + v \cdot \nabla s_{{\text{ksc}}} - D_v \nabla ^2 s_{{\text{ksc}}} = 0$$(20)$${\text{RPE: }}\frac{{\partial s_{{\text{ksc}}} }}{{\partial t}} + \left( {v + k_{{\text{act}}} } \right) \cdot \nabla s_{{\text{ksc}}} - D_{{\text{rpe}}} \nabla ^2 s_{{\text{ksc}}} = 0$$(21)$${\text{CS: }}\frac{{\partial s_{ksc} }}{{\partial t}} + \left( {v + k_{{\text{act}}} } \right) \cdot \nabla s_{{\text{ksc}}} - D_{{\text{rpe}}} \nabla ^2 s_{{\text{ksc}}} = 0$$(22) -
2.
Boundary conditions
$${\text{Lens: }}n \cdot \left( { - D\nabla s_{{\text{ksc}}} + vs_{{\text{ksc}}} } \right) = 0$$(23)$${\text{Hyaloid: }}n \cdot \left( { - D\nabla s_{{\text{ksc}}} + vs_{{\text{ksc}}} } \right) = k_{{\text{hy}}} s_{{\text{ksc}}} $$(24)$${\text{Sclera: }}n \cdot \left( { - D\nabla s_{{\text{ksc}}} + vs_{{\text{ksc}}} } \right) = k_{{\text{sc}}} s_{{\text{ksc}}} + c$$(25)
The average values of s γ and s ksc over the vitreous volume were calculated and used for the Gauss–Newton curve fitting algorithm.
Rights and permissions
About this article
Cite this article
Balachandran, R.K., Barocas, V.H. Computer Modeling of Drug Delivery to the Posterior Eye: Effect of Active Transport and Loss to Choroidal Blood Flow. Pharm Res 25, 2685–2696 (2008). https://doi.org/10.1007/s11095-008-9691-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9691-3