Abstract
Purpose
The aim of this paper is to enhance the dissolution rate of racemic (R,S)-(±)-sodium ibuprofen dihydrate via a bio-inspired method of growing mesocrystals.
Materials and Methods
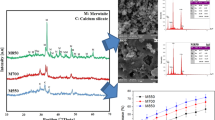
Mesocrystals of racemic (R,S)-(±)-sodium ibuprofen dihydrate were successfully prepared from a supersaturated aqueous solution of racemic (R,S)-(±)-sodium ibuprofen dihydrate having the initial degree of supersaturation, S 0 , of 1.326 and the initial saturated concentration, C*, of 0.986 mol/l at 25°C with sodium dodecyl sulfate (SDS) at a concentration of 0.10 g/l. Dynamic light scattering, scanning electron microscopy, powder X-ray diffraction, differential scanning calorimetry, and optical microscopy with cross polarizers were employed to understand the formation mechanism and to characterize the superstructures of the SDS generated mesocrystals.
Results
The SDS generated mesocrystals were the assembly of the oriented attachment of racemic (R,S)-(±)-sodium ibuprofen dihydrate nano-sized platelets under the mediation of the side-to-side interaction between SDS and racemic (R,S)-(±)-sodium ibuprofen dihydrate. The SDS generated mesocrystals contained a mixture of the racemic compounds in α- and β-forms and the resolved racemic conglomerate in γ-form with no detectable amount of SDS. The dissolution rate of the SDS generated mesocrystals was more rapid than the one of its counterpart made by conventional crystallization pathway.
Conclusions
The crystallization of racemic (R,S)-(±)-sodium ibuprofen dihydrate in the presence of SDS yielded well-faceted, well-separated, but almost perfectly three-dimensionally aligned nano-sized platelets. This kind of bio-inspired mesocrystal superstructure has definitely opened a new doorway for crystal engineering and pre-formulation design in pharmaceutical industry. The future work is to study the mesocrystal formation of some other active pharmaceutical ingredients in organic solvent systems and to develop an efficient method for screening the additives.









Similar content being viewed by others
References
L. J. Sellars. Special report: Executive prophecies—pharmaceuticals in the new millennium. Pharm. Exec. 60–72 (January 2002).
Ö. Almarsson, and M. J. Zaworotko. Crystal engineering of the composition of pharmaceutical phases. Do pharmaceutical co-crystals represent a new path to improved medicines. Chem. Commun. 17:1889–1896 (2004).
Trends in the Pharmaceutical Industry. www.patheon.com/overview/trends.html.
R. J. Bastin, M. J. Bowker, and B. J. Slater. Salt selection and optimisation procedures for pharmaceutical new chemical entities. Org. Proc. Res. Dev 4(5):427–435 (2000).
S. N. Black, E. A. Collier, R. J. Davey, and R. J. Roberts. Structure, solubility, screening, and synthesis of molecular salts. J. Pharm. Sci 96(5):1053–1068 (2007).
L. R. Hilden, and K. R. Morris. Physics of amorphous solids. J. Pharm. Sci 93(1):3–12 (2004).
R. Hilfiker, J. Berghausen, F. Blatter, A. Burkhard, S. M. De Paul, B. Freiermuth, A. Geoffroy, U. Hofmeier, C. Marcolli, B. Siebenhaar, M. Szelagiewicz, A. Vit, and M. von Raumer. Polymorphism – Integrated approach from high-throughput screening to crystallization optimization. J. Therm. Anal. Calor 73(2):429–440 (2003).
N. Blagden, and R. J. Davey. Polymorph selection: Challenges for the future? Cryst. Growth Des 3(6):873–885 (2003).
T. Lee, S. T. Hung, and C. S. Kuo. Polymorph farming of acetaminophen and sulfathiazole on a chip. Pharm. Res 23(11):2542–2555 (2006).
D. Singhal, and W. Curatolo. Drug polymorphism and dosage form design: A practical perspective. Adv. Drug Deliv. Rev 56(3):335–347 (2004).
P. Vishweshwar, J. A. McMahon, M. L. Peterson, M. B. Hickey, T. R. Shattock, and M. J. Zaworotko. Crystal engineering of pharmaceutical co-crystals from polymorphic active pharmaceutical ingredients. Chem. Commun. 36:4601–4603 (2005).
S. L. Morissette, Ö. Almarsson, M. L. Peterson, J. F. Remenar, M. J. Read, A. V. Lemmo, S. Ellis, M. J. Cima, and C. R. Gardner. High-throughput crystallization: polymorphs, salts, co-crystals and solvates of pharmaceutical solids. Adv. Drug Deliv. Rev 56(3):275–300 (2004).
H. Koshima, and M. Miyauchi. Polymorphs of a cocrystal with achiral and chiral structures prepared by pseudoseeding: tryptamine/hydrocinnamic acid. Cryst. Growth Des 1(5):355–357 (2001).
G. G. Z. Zhang, R. F. Henry, T. B. Borchardt, and X. Lou. Efficient co-crystal screening using solution-mediated phase transformation. J. Pharm. Sci 96(5):990–995 (2007).
A. M. Thayer. Form and Function. C&EN, 17–30 (June 18 2007).
A. T. M. Serajuddin. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J. Pharm. Sci 88(10):1058–1066 (1999).
T. Lee, and J. Lee. Drug-carrier screening on a chip. Pharm. Tech 27(1):40–48 (2003).
J.-F. Chen, M.-Y. Zhou, L. Shao, Y.-Y. Wang, J. Yun, N. Y. K. Chew, and H-K. Chan. Feasibility of preparing nanodrugs by high-gravity reactive precipitation. Inter. J. Pharm 269(1):267–274 (2004).
S. X. Yin, M. Franchini, J. Chen, A. Hsieh, S. Jen, T. Lee, M. Hussain, and R. Smith. Bioavailability enhancement of a COX-2 inhibitor, BMS-347070, from a nanocrystalline dispersion prepared by spray-drying. J. Pharm. Sci. 94(7):1598–1607.
N. A. Peppas. Intelligent therapeutics: biomimetic systems and nanotechnology in drug delivery. Adv. Drug Deliv. Rev 56(11):1529–1531 (2004).
P. Van Arnum. Nanotechnology advances in drug delivery. Pharm. Tech 31(6):48–52 (2007).
I. Soten, and G. A. Ozin. New directions in self-assembly: materials synthesis over “all” length scales. Curr. Opin. Colloid Inter. Sci 4(5):325–337 (1999).
Y. Oaki, and H. Imai. Hierarchially organized superstructure emerging from the exquisite association of inorganic crystals, organic polymers, and dyes: a model approach towards suprabiomineral materials. Adv. Funct. Mater 15(9):1407–1414 (2005).
A.-W. Xu, Y. Ma, and H. Cölfen. Biomimetic mineralization. J. Mater. Chem 17(5):415–449 (2007).
H. Cölfen, and M. Antonietti. Mesocrystals: inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. Int. Ed 44(35):5576–5591 (2005).
H. Cölfen, and S. Mann. Higher-order organization by mesoscale self-assembly and transformation of hybrid nanostructures. Angew. Chem. Int. Ed 42(20):2350–2365 (2003).
Y. Ma, H. Cölfen, and M. Antonietti. Morphosynthesis of alanine mesocrystals by pH control. J. Phys. Chem. B 110(22):10822–10828 (2006).
C.-M. Chun. Hydrothermal crystallization of barium titanate: mechanisms of nucleation and growth. Ph. D. Dissertation, Department of Geosciences, Princeton University, June 1997.
I. Katzhendler, R. Azoury, and M. Friedman. Crystalline properties of carbamazepine in sustained release hydrophilic matrix tablets based on hydroxypropyl methylcellulose. J. Control. Release 54(1):69–85 (1998).
S. Wohlrab, N. Pinna, M. Antonietti, and H. Cölfen. Polymer-induced alignment of DL- alanine nanocrystals to crystalline mesostructures. Chem. Eur. J 11(10):2903–2913 (2005).
B. J. Armitage, J. F. Lampard, and A. Smith. Composition of S-Sodium ibuprofen. US Patent 6,242,000 B1 (2001).
T. Lee, Y. H. Chen, and Y. W. Wang. Effects of homochiral molecules of (S)-(+)-ibuprofen and (S)-(-)-sodium ibuprofen dihydrate on the crystallization kinetics of racemic (R,S)-(±)-sodium ibuprofen dihydrate. Cryst. Growth Des. 8(2):415–426 (2008).
Y. Zhang, and D. J. W. Grant. Similarity in structures of racemic and enantiomeric ibuprofen sodium dihydrates. Acta Cryst. C61(9):m435–m438 (2005).
G. G. Z. Zhang, S. Y. L. Paspal, R. Suryanarayanan, and D. J. W. Grant. Racemic species of sodium ibuprofen: characterization and polymorphic relationships. J. Pharm. Sci 92(7):1356–1366 (2003).
S. Behn. Sodium lauryl sulfate. In A. Wade, and P. J. Weller (eds.), Handbook of Pharmaceutical Excipients, 2American Pharmaceutical Association, Washington, USA, 1994, pp. 448–450.
Y. Xiong, Y. Xie, J. Yang, R. Zhang, C. Wu, and G. Du. In situ micelle-template-interface reaction route to CdS nanotubes and nanowires. J. Mater. Chem 12(12):3712–3716 (2002).
N. Jongen, P. Bowen, J. Lemaītre, J.-C. Valmalette, and H. Hofmann. Precipitation of Self-organized copper oxalate polycrystalline particles in the presence of hydroxypropylmethylcellulose (HPMC): control of morphology. J. Colloid Inter. Sci 226(2):189–198 (2000).
W. Sorasuchart, J. Wardrop, and J. W. Ayres. Drug release from spray layered and coated drug-containing beads: effects of pH and comparison of different dissolution methods. Drug Dev. Ind. Pharm 25(10):1093–1098 (1999).
A. T. M. Serajuddin. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev 59(7):603–616 (2007).
A. Ridell, H. Evertsson, S. Nilson, and L. Sundelöf. Amphiphilic association of ibuprofen and two nonionic cellulose derivatives in aqueous solution. J. Pharm. Sci 88(11):1175–1181 (2000).
A. L. D. Vries, and T. J. Price. Role of glycopeptides and peptides in inhibition of crystallization of water in polar fishes. Phil. Trans. R. Soc. Lond. B 304(1121):575–588 (1984).
H. P. Klug, and L. E. Alexander. Crystallite size and lattice strains from line broadening. Chapter 9 in X-ray Diffraction Procedures, 2nd ed., Wiley, New York, 1974, pp. 657–661.
Acknowledgements
This work was supported by a grant from the National Science Council of Taiwan, Republic of China (NSC 95-2113-M-008-012-MY2). Assistance from Ms. Jui-Mei Huang in DSC, Ms. Shew-Jen Weng in PXRD, and Ms. Ching-Tien Lin for SEM and EDS, and all with the Precision Instrument Center and High Valued Instrument Center at National Central University are gratefully acknowledged. We also thank the assistance from Ms. Yi-Yin Lai in DLS with Molecular BioEngineering Laboratory, and my four other students, Mr. Hsiang-Yu Hsieh, Mr. Yeh-Wen Wang, Mr. Hung-Ju Hou, and Mr. Yan-Chan Su in collecting SEM, DLS and dissolution data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, T., Zhang, C.W. Dissolution Enhancement by Bio-Inspired Mesocrystals: The Study of Racemic (R,S)-(±)-Sodium Ibuprofen Dihydrate. Pharm Res 25, 1563–1571 (2008). https://doi.org/10.1007/s11095-008-9554-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9554-y