Abstract
Introduction
The human organic anion transporting polypeptide C (OATPC) is one of the major transport proteins involved in the enterohepatic circulation of bile salts and plays an important role in vectorial transport of organic anions and drugs across hepatocytes.
Materials and Methods
In this study, the effects of biological reagents on the membrane localization of OATPC were investigated by confocal microscopy and estrone-3-sulfate transport.
Results
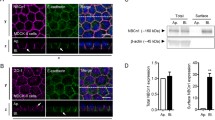
Our results demonstrated that the functional membrane expression of fluorescent chimera OATPC-GFP was achieved in non-polarized (COS7 and HEK293) and polarized (MDCK) cells. Both brefeldin A (a Golgi complex disruptor) and bafilomycin A1 (an inhibitor of vacuolar H+-ATPase) treatment significantly decreased the polarized membrane trafficking and markedly reduced the uptake of estrone-3-sulfate (∼40–90%) in OATPC-GFP transfected cells, suggesting that membrane sorting of hOATPC-GFP was mediated by Golgi complex and vacuolar H+-ATPase-related vesicle transport pathways. Treatment with 8-Br-cAMP (a cAMP analog) stimulated OATPC-GFP membrane localization and enhanced estrone-3-sulfate uptake by ∼20%. The protein kinase A (PKA) inhibitors (H89 and KT5720), but not a PKG inhibitor, blocked the polarized membrane expression of OATPC-GFP and reduced estrone-3-sulfate transport activity. The simultaneous treatment of cells with PKA activator/inhibitor and bafilomycin A1 demonstrated that bafilomycin A1 did not change the effects of 8-Br-cAMP and H89 on the membrane localization of OATPC-GFP compared with the use of 8-Br-cAMP and H89 alone.
Discussion
These data suggest that a cAMP-PKA sensitive membrane sorting pathway for OATPC-GFP is independent of the vacuolar H+-ATPase associated (bafilomycin A1 sensitive) vesicle mediated membrane sorting pathway. In contrast, with combined treatment with brefeldin A, neither the PKA-activator (8-Br-cAMP) nor the inhibitor (H89) further altered the plasma membrane expression and transport activity of OATPC-GFP compared with brefeldin A treatment alone. These data suggest that the cAMP-PKA regulation of OATPC membrane expression involves the Golgi complex. When the Golgi apparatus was disrupted by brefeldin A treatment, the effects of cAMP-PKA on the Golgi-to-basolateral surface sorting process of OATPC was also diminished. In summary, the plasma membrane localization of human OATPC is mediated by Golgi complex and vacuolar H+-ATPase vesicle mediated membrane sorting pathways. cAMP-PKA regulates sorting process through the Golgi complex but not the vacuolar H+-ATPase associated vesicular pathway.









Similar content being viewed by others
Abbreviations
- BA1:
-
bafilomycin A1
- BFA:
-
brefeldin A
- E3S:
-
Estrone-3-sulfate
- GFP:
-
green fluorescent protein
- H89:
-
N-[2-(methylamino)ethyl]-5-isoquinolinesulfonamide
- OATP-GFP:
-
GFP-fused human OATPC
- OATP:
-
human organic anion transporting polypeptide-C
- PKGi:
-
protein kinase G inhibitor
- 8-Br-cAMP:
-
8-bromoadenosine-3′,5′-cyclic monophosphate
References
K. Ito, H. Suzuki, T. Horie, and Y. Sugiyama. Apical/basolateral surface expression of drug transporters and its role in vectorial drug transport. Pharm. Res. 22(10):1559–1577 (2005).
M. G. Roma, P. Milkiewicz, E. Elias, and R. Coleman. Control by signaling modulators of the sorting of canalicular transporters in rat hepatocyte couplets: role of the cytoskeleton. Hepatology 32(6):1342–1356 (2000).
P. Wang, J. J. Wang, Y. Xiao, J. W. Murray, P. M. Novikoff, R. H. Angeletti, G. A. Orr, D. Lan, D. L. Silver, and A. W. Wolkoff. Interaction with PDZK1 is required for expression of organic anion transporting protein 1A1 on the hepatocyte surface. J. Biol. Chem. 280(34):30143–30149 (2005).
R. G. Tirona, B. F. Leake, G. Merino, and R. B. Kim. Polymorphisms in OATP-C. Identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J. Biol. Chem. 276:35669–35675 (2001).
C. Michalski, Y. Cui, A. T. Nies, A. K. Nuessler, P. Neuhaus, U. M. Zanger, K. Klein, M. Eichelbaum, D. Keppler, and J. König. A naturally occurring mutation in the SLC21A6 gene causing impaired membrane localization of the hepatocyte uptake transporter. J. Biol. Chem. 277:43058–43063 (2002).
A-Q. Sun, M. A. Arresa, L. Zeng, I’. K. Swaby, M. M. Zhou, and F. J. Suchy. The rat liver Na+/bile acid cotransporters (Ntcp): importance of the cytoplasmic tail to function and plasma membrane targeting. J. Biol. Chem. 276:6825–6833 (2001).
A-Q. Sun, M. Ananthanarayanan, C. J. Soroka, S. Thevananther, B. Shneider, and F. J. Suchy. Sorting of the rat liver and ileal sodium-dependent bile acid transporters in polarized epithelial cells. Am. J. Physiol. Gasterointest. Liver Physiol. 275:G1045–G1055 (1998).
M. J. Lee, J. Y. Park, S. Y. Lee, J. S. Lee, D. K. Jung, Y. S. Bae, and J. Y. Kwak. Modulation of constitutive and delayed apoptosis by brefeldin A in human neutrophils. Int. Immunopharmacol. 3(6):835–843 (2003).
G. Arreaza and D. A. Brown. Sorting and intracellular trafficking of a glycosylphosphatidylinositol-anchored protein and two hybrid transmembrane proteins with the same ectodomain in Madin–Darby canine kidney epithelial cells. J. Biol. Chem. 270:23641–23647 (1995).
A-Q. Sun, N. Balasubramaniyan, C-J. Liu, M. Shahid, and F. J. Suchy. Association of the 16 kDa subunit C of vacuolar proton pump with ileal Na+-dependent bile acid transporter: protein–protein interaction and intracellular trafficking. J. Biol. Chem. 279:16295–16300 (2004).
E. A. Potter, G. Stewart, and C. P. Smith. Urea flux across MDCK-mUT-A2 monolayers is acutely sensitive to AVP, cAMP, and [Ca2+]i. Am. J. Physiol. Renal. Physiol. 291(1):F122–F128 (2006).
T. Kagawa, L. Varticovski, Y. Sai, and I. M. Arias. Mechanism by which cAMP activates PI3-kinase and increases bile acid secretion in WIF-B9 cells. Am. J. Physiol. Cell Physiol. 283(6):C1655–C1666 (2002).
D. B. Glass. Differential responses of cyclic GMP-dependent and cyclic AMP-dependent protein kinases to synthetic peptide inhibitors. Biochem. J. 213(1):159–164 (1983).
S. Puri and A. D. Linstedt. Capacity of the golgi apparatus for biogenesis from the endoplasmic reticulum. Mol. Biol. Cell 14(12):5011–5018 (2003).
A-Q. Sun, N. Balasubramaniyan, K. Xu, C. J. Liu, V. M. Ponamgi, H. Liu, and F. J. Suchy. Protein–protein interaction and membrane localization of human organic solute transporter (hOST). Am. J. Physiol. Gasterointest. Liver Physiol. 292:G1586–G1593 (2007).
L. B. Goh, K. J. Spears, D. Yao, A. Ayrton, P. Morgan, W. C. Roland, and T. Friedberg. Endogenous drug transporters in in vitro and in vivo models for the prediction of drug disposition in man. Biochem. Pharmacol. 64:1569–1578 (2002).
Y. J. Hei, K. L. MacDonell, J. H. McNeill, and J. Diamond. Lack of correlation between activation of cyclic AMP-dependent protein kinase and inhibition of contraction of rat vas deferens by cyclic AMP analogs. Mol. Pharmacol. 39(2):233–238 (1991).
R. Safaei, K. Katano, B. J. Larson, G. Samimi, A. K. Holzer, W. Naerdemann, M. Tomioka, M. Goodman, and S. B. Howell. Intracellular localization and trafficking of fluorescein-labeled cisplatin in human ovarian carcinoma cells. Clin. Cancer Res. 11(2 Pt 1):756–767 (2005).
B. Kristensen, S. Birkelund, and P. L. Jorgensen. Trafficking of Na,K-ATPase fused to enhanced green fluorescent protein is mediated by protein kinase A or C. J. Membr. Biol. 191(1):25–36 (2003).
A-Q. Sun, I’. K. Swaby, S-H. Xu, and F. J. Suchy. Cell specific basolateral membrane sorting of the human liver Na+-dependent bile acid cotransporter. Am. J. Physiol. 280:G1305–G1313 (2001).
G. A. Kullak-Ublick, B. Stieger, and P. J. Meier. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology 126(1):322–342 (2004).
M. Sasaki, H. Suzuki, K. Ito, T. Abe, and Y. Sugiyama. Transcellular transport of organic anions across a double-transfected Madin–Darby canine kidney II cell monolayer expressing both human organic anion-transporting polypeptide (OATP2/SLC21A6) and multidrug resistance-associated protein 2 (MRP2/ABCC2). J. Biol. Chem. 277:6497–6503 (2002).
K. A. Wojtal, E. de Vries, D. Hoekstra, and S. C. van Ijzendoorn. Efficient trafficking of MDR1/P-glycoprotein to apical canalicular plasma membranes in HepG2 cells requires PKA-RIIalpha anchoring and glucosylceramide. Mol. Biol. Cell 17(8):3638–3650 (2006).
J. D. Smith, M. Miyata, M. Ginsberg, C. Grigaux, E. Shmookler, and A. S. Plump. Cyclic AMP induces apolipoprotein E binding activity and promotes cholesterol efflux from a macrophage cell line to apolipoprotein acceptors. J. Biol. Chem. 271(48):30647–30655 (1996).
N. L. Nakhoul and L. L. Hamm. Vacuolar H(+)-ATPase in the kidney. J. Nephrol. 5:S22–S31 (2002).
M. Forgac. Structure, mechanism and regulation of the clathrin-coated vesicle and yeast vacuolar H(+)-ATPases. J. Exp. Biol. 203(Pt 1):71–80 (2000).
T. Nishi and M. Forgac. The vacuolar (H+)-ATPases—nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 3(2):94–103 (2002).
B. A. Guillet, L. J. Velly, B. Canolle, F. M. Masmejean, A. L. Nieoullon, and P. Pisano. Differential regulation by protein kinases of activity and cell surface expression of glutamate transporters in neuron-enriched cultures. Neurochem. Int. 46(4):337–346 (2005).
Z. B. Pristupa, F. McConkey, F. Liu, H. Y. Man, F. J. Lee, Y. T. Wang, and H. B. Niznik. Protein kinase-mediated bidirectional trafficking and functional regulation of the human dopamine transporter. Synapse 30(1):79–87 (1998).
J. Zhou, J. Yi, N. Hu, A. L. George Jr, and K. T. Murray. Activation of protein kinase A modulates trafficking of the human cardiac sodium channel in Xenopus oocytes. Circ. Res. 87(1):33–38 (2000).
S. Y. Chang, A. Di, A. P. Naren, H. C. Palfrey, K. L. Kirk, and D. J. Nelson. Mechanisms of CFTR regulation by syntaxin 1A and PKA. J. Cell Sci. 115(Pt 4):783–791 (2002).
J. Yao, J. D. Erickson, and L. B. Hersh. Protein kinase A affects trafficking of the vesicular monoamine transporters in PC12 cells. Traffic 5(12):1006–1016 (2004).
M. B. Butterworth, R. A. Frizzell, J. P. Johnson, K. W. Peters, and R. S. Edinger. PKA-dependent ENaC trafficking requires the SNARE-binding protein complexin. Am. J. Physiol. Renal. Physiol. 289(5):F969–F977 (2005).
P. V. Burgos, C. Klattenhoff, E. de la Fuente, A. Rigotti, and A. Gonzalez. Cholesterol depletion induces PKA-mediated basolateral-to-apical transcytosis of the scavenger receptor class B type I in MDCK cells. Proc. Natl. Acad. Sci. U. S. A. 101(11):3845–3850 (2004).
T. M. Lincoln, T. L. Cornwell, and A. E. Taylor. cGMP-dependent protein kinase mediates the reduction of Ca2ı by cAMP in vascular smooth muscle cells. Am. J. Physiol. 258:C399–C407 (1990).
T. L. Cornwell and T. M. Lincoln. Regulation of intracellular Ca’ı levels in cultured vascular smooth muscle cells. J. Biol. Chem. 264:1146–1155 (1989).
S. G. Francis, B. D. Nobelett, B. W. Todd, J. N. Wells, and J. D. Corbin. Relaxation of vascular tracheal smooth muscle by cyclic nucleotide analogs that preferentially activate purified cGMP-dependent protein kinase. Mol. Pharmacol. 34:506–517 (1988).
J. F. Kuo, M. Shoji, and W. N. Kuo. Molecular and physiological aspects of mammalian cyclic GMP-dependent protein kinase. Annu. Rev. Pharmacol. Toxicol. 18:341–356 (1978).
F. Golin-Bisello, N. Bradbury, and N. Ameen. STa and cGMP stimulate CFTR translocation to the surface of villus enterocytes in rat jejunum and is regulated by protein kinase G. Am. J. Physiol. Cell Physiol. 289(3):C708–C716 (2005).
E. Bejarano, M. Cabrera, L. Vega, J. Hidalgo, and A. Velasco. Golgi structural stability and biogenesis depend on associated PKA activity. J. Cell Sci. 119(Pt 18):3764–3775 (2006).
C. P. Thomas, J. R. Campbell, P. J. Wright, and R. F. Husted. cAMP-stimulated Na+ transport in H441 distal lung epithelial cells: role of PKA, phosphatidylinositol 3-kinase, and sgk1. Am. J. Physiol. Lung Cell Mol. Physiol. 287(4):L843–L851 (2004).
Y. Marunaka and N. Niisato. H89, an inhibitor of protein kinase A (PKA), stimulates Na+ transport by translocating an epithelial Na+ channel (ENaC) in fetal rat alveolar type II epithelium. Biochem. Pharmacol. 66(6):1083–1089 (2003).
Acknowledgments
The authors gratefully acknowledge Dr. Wen-Sheng Chen (Yale Liver Center Yale University School of Medicine New Haven CT) for the initial amplification of the human OATPC cDNA. This work was supported in part by the National Institutes of Health Grants 5R37HD020632-21 (to F. J. S.), DK 25636 (to J.L.B.), and the DK P30-34989 (to Yale Liver Center). Confocal laser scanning microscopy was performed at the MSSM-CLSM core facility supported with funding from the NIH-NCI shared resources grant (5R24 CA095823-04), NSF Major Research Instrumentation grant (DBI-9724504), and NIH shared instrumentation grant (1 S10 RR0 9145-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, AQ., Ponamgi, V.M., Boyer, J.L. et al. Membrane Trafficking of the Human Organic Anion-Transporting Polypeptide C (hOATPC). Pharm Res 25, 463–474 (2008). https://doi.org/10.1007/s11095-007-9399-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9399-9