Abstract
Purpose
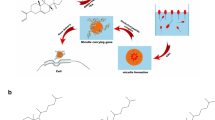
Cationic polymers (i.e. polyallylamine, poly-L-lysine) having primary amino groups are poor transfection agents and possess high cytotoxicity index when used without any chemical modification and usually entail specific receptor mediated endocytosis or lysosomotropic agents to execute efficient gene delivery. In this report, primary amino groups of polyallylamine (PAA, 17 kDa) were substituted with imidazolyl functions, which are presumed to enhance endosomal release, and thus enhance its gene delivery efficiency and eliminate the requirement of external lysosomotropic agents. Further, systems were cross-linked with polyethylene glycol (PEG) to prepare PAA-IAA-PEG (PIP) nanoparticles and evaluated them in various model cell lines.
Materials and Methods
The efficacy of PIP nanoparticles in delivering a plasmid encoding enhanced green fluorescent protein (EGFP) gene was assessed in COS-1, N2a and HEK293 cell lines, while their cytotoxicity was investigated in COS-1 and HEK293 cell lines. The PAA was chemically modified using imidazolyl moieties and ionically cross-linked with PEG to engineer nanoparticles. The extent of substitution was determined by ninhydrin method. The PIP nanoparticles were further characterized by measuring the particle size (dynamic light scattering and transmission electron microscopy), surface charge (zeta potential), DNA accessibility and buffering capacity. The cytotoxicity was examined using the MTT method.
Results
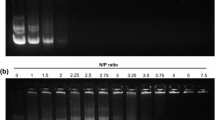
In vitro transfection efficiency of synthesized nanoparticles is increased up to several folds compared to native polymer even in the presence of serum, while maintaining the cell viability over 100% in COS-1 cells. Nanoparticles possess positive zeta potential between 5.6–13 mV and size range of 185–230 nm in water. The accessibility experiment demonstrated that nanoparticles with higher degree of imidazolyl substitution formed relatively loose complexes with DNA. An acid-base titration showed enhanced buffering capacity of modified PAA.
Conclusions
The PIP nanoparticles reveal tremendous potential as novel delivery system for achieving improved transfection efficiency, while keeping the cells at ease.










Similar content being viewed by others
Change history
03 December 2020
A Correction to this paper has been published: https://doi.org/10.1007/s11095-020-02971-0
References
E. Piskin, S. Dincer, and M. Turk. Gene delivery: intelligent but just at the beginning. J. Biomater. Sci., Polym. Ed.15:1181–1202 (2004).
D. Luo and W. M. Saltzman. Synthetic DNA delivery systems. Nat. Biotechnol.18:33–37 (2000).
C. M. Cavazzana, S. B. Hacein, G. B. deSaint, F. Gross, E. Yvon, P. Nusbaum, P. Selz, C. Hue, S. Certain, J. L. Casanova, P. Bousso, F. L. Deist, and A. Fischer. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science288:669–672 (2000).
C. M. Liu, D. P. Liu, W. J. Dong, and C.-C. Liang. Retrovirus vector-mediated stable gene silencing in human cell. Biochem. Biophys. Res. Commun.313:716–720 (2004).
D. T. Curiel, S. Agrawal, E. Wagner, and M. Cotton. Adenovirus enhancement of transferrin-polylysine-mediated gene delivery. Proc. Natl. Acad. Sci. U.S.A.88:8850–8854 (1991).
A. Fasbender, J. Zabner, M. Chillon, T. O. Moninger, A. P. Puga, B. L. Davidson, and M. J. Welsh. Complexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vitro and in vivo. J. Biol. Chem.272:6479–6489 (1997).
P. L. Felgner, T. R. Gadek, M. Holm, R. Roman, H. W. Chan, M. Wenz, J. P. Northrop, G. M. Ringold, and M. Danielsen. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. U.S.A.84:7413–7417 (1987).
M. A. Ilies, B. H. Johnson, F. Makori, A. Miller, W. A. Seitz, F. B. Thompson, and A. T. Balaban. Pyridinium cationic lipids in gene delivery: an in vitro and in vivo comparison of transfection efficiency versus a tetraalkylammonium congener. Arch. Biochem. Biophys.435:217–226 (2005).
S. Simoes, A. Filipe, H. Faneca, M. Mano, N. Penacho, N. Duzgunes, and M. P. de Lima. Cationic liposomes for gene delivery. Expert Opin. Drug Deliv.2:237–254 (2005).
S. I. Kim, S. K. Lee, Y. M. Park, Y. B. Lee, S. C. Shin, K. C. Lee, and I. J. Oh. Physicochemical characterization of poly(l-lactic acid) and poly(d,l-lactide-co-glycolide) nanoparticles with polyethylenimine as gene delivery carrier. Int. J. Pharm.298:255–262 (2005).
R. G. Crystal. Transfer of genes to human: early lessons and obstacles to success. Science270:404–410 (1995).
P. L. Felgner, Y. Barenholz, J. P. Behr, S. H. Cheng, P. Cullis, L. Huang, J. A. Jessee, L. Seymour, F. Szoka, A. R. Thierry, E. Wagner, and G. Wu. Nomenclature for synthetic gene delivery systems. Hum. Gene Ther.20:511–512 (1997).
S. Hacein-Bey-Abina, C. Von Kalle, M. Schmidt, M. P. McCormack, N. Wulffraat, P. Leboulch, A. Lim, C. S. Osborne, R. Pawliuk, E. Morillon, R. Sorensen, A. Forster, P. Fraser, J. I. Cohen, G. de Saint Basile, I. Alexander, U. Wintergerst, T. Frebourg, A. Aurias, D. Stoppa-Lyonnet, S. Romana, I. Radford-Weiss, F. Gross, F. Valensi, E. Delabesse, E. Macintyre, F. Sigaux, J. Soulier, L. E. Leiva, M. Wissler, C. Prinz, T. H. Rabbitts, F. Le Deist, A. Fischer, and M. Cavazzana-Calvo. LMO2-associated clonal T-cell proliferation in two patients after gene therapy for SCID-X1. Science302:400–401(2003).
J.-P. Behr. The proton sponge: a trick to enter cells the viruses did not exploit. Chimia.51:34–36 (1997).
P. Chollet, M. C. Favrot, A. Hurbin, and J. L. Coll. Side-effects of a systemic injection of linear polyethylenimine–DNA complexes. J. Gene Med.4:84–91(2002).
W. T. Godbey, K. K. Wu, and A. G. Mikos. Poly(ethylenimine) and its role in gene delivery. J. Control. Release60:149–160 (1999).
S. Nimesh, R. Kumar, and R. Chandra. Novel polyallylamine–dextran sulfate–DNA nanoplexes: highly efficient non-viral vector for gene delivery. Int. J. Pharm.320:143–149 (2006).
O. Boussif, T. Delair, C. Brua, L. Veron, A. Pavirani, and H. V. Kolbe, O. Synthesis of polyallylamine derivative and their use as a gene transfer vectors in vitro. Bioconjug. Chem.10:877–883 (1999).
Y. H. Choi, F. Liu, J. S. Kim, Y. K. Choi, J. S. Park, and S. W. Kim. Polyethylene glycol-grafted-polylysine as polymeric gene carriers. J. Control. Release54:39–48 (1998).
M. T. Peracchia, C. Vauthier, D. Desmaele, A. Gulik, C. Dedieu, M. demoy, J. Angelo, and P. Couvreur. Pegylated nanoparticles from a novel methoxypolyethylene-glycol cyanoacrylate-hexadecyl amphiphile copolymer. Pharm. Res.15:550–556 (1990).
C. H. Ahn, S. Y Chae, Y. H. Bae, and S. W. Kim. Synthesis of biodegradable multi-block copolymers of poly(L-lysine) and poly(ethylene glycol) as a non-viral gene carrier. J. Control. Release97:567–574 (2004).
M. L. Forrest, G. E. Meister, J. T. Koerber, and D. W. Pack. Partial acetylation of polyethylenimine enhances in vitro gene delivery. Pharm. Res.21:365–371 (2004).
W. Tiyaboonchai, J. Woiszwillo, and C. R. Middaugh. Formulation and characterization of DNA–polyethyleneimine-dextran sulfate nanoparticles. Eur. J. Pharm. Sci.19:191–202 (2003).
M. L. Forrest, N. Gabrielson, and D. W. Pack. Cyclodextrin-polyethylenimine conjugates for targeted in vitro gene delivery. Biotechnol. Bioeng.89:416–423(2005).
D. J. Chen, B. S. Majors, A. Zelikin, and D. Putnam. Structure-function relationship of gene delivery vectors in a limited polycation library. J. Control. Release103:273–293 (2005).
D. W. Pack, D. Putnam, and R. Langer. Design of imidazole-containing endosomolytic biopolymers for gene delivery. Biotechnol. Bioeng.67:217–223 (2000).
R. M. Bello, and P. Midoux. Histidylated polylysine as DNA vector: elevation of the imidazole protonation and reduced cellular uptake without change in the polyfection efficiency of serum stabilized negative polyplexes. Bioconjug. Chem.12:92–99 (2001).
P. Dubruel, B. Christiaens, M. Rosseneu, J. Vandekerckhove, J. Grooten, V. Goossens, and E. Schacht. Buffering properties of cationic polymethacrylates are not the only key to successful gene delivery. Biomacromolecules5:379–388 (2004).
T. H. Kim, J. E. Ihm, Y. J. Choi, J. W. Nah, and C. S. Cho. Efficient gene delivery by urocanic acid-modified chitosan. J. Control. Release93:389–402 (2003).
A. Swami, A. Aggarwal, A. Pathak, S. Patnaik, P. Kumar, Y. Singh, and K. C. Gupta. Imidazolyl-PEI modified nanoparticles for enhanced gene delivery. Int. J. Pharm. (2007) (In press).
J. Suh, H.-J. Paik, and B. K. Hwang. Ionization of Poly(ethylene) and Poly(allylamine) at various pH’s. Bioorg. Chem.22:318–327 (1994).
H. Eliyahu, A. Makovitzki, T. Azzam, A. Zlotkin, A. Joseph, D. Gazit, Y. Barenholz, and A. J. Domb. Novel dextran-spermine conjugates as transfecting agents: comparing water-soluble and micellar polymers. Gene Ther.12:494–503 (2005).
S. Nimesh, A. Goyal, V. Pawar, S. Jayaraman, P. Kumar, R. Chandra, Y. Singh, and K. C. Gupta. Polyethylenimine nanoparticles as efficient transfecting agents for mammalian cells. J. Control. Release110:457–468 (2006).
P.-Y. Yeh, P. Kopeckova, and J. Kopecek. Biodegradable and pH sensitive hydrogels: synthesis by crosslinking of N,N-dimethylacrylamide copolymer precursors. J. Polym. Sci., A, Polym. Chem.32:1627–1637 (1994).
D. Putnam, C. A. Gentry, D. W. Pack, and R. Langer. Polymer-based gene delivery with low cytotoxicity by a unique balance of side-chain termini. Proc. Natl. Acad. Sci. U.S.A.98:1200–205 (2001).
M. Glodde, S. R. Sirsi, and G. J. Lutz. Physiochemical properties of low and high molecular weight poly(ethylene glycol)-grafted poly(ethyleneimine) copolymers and their complexes with oligonucleotides. Biomacromolecules7:347–356 (2006).
M. B. Roufai, and P. Midoux. Histidylated polylysine as DNA vector: elevation of the imidazole protonation and reduced cellular uptake without change in the polyfection efficiency of serum stabilized negative polyplexes. Bioconjug. Chem.12:92–99 (2001).
J. E. Ihm, Ki-Ok Han, C. S. Hwang, J. H. Kang, K.-D. Ahn, I.-K. Han, D. K. Han, J. A. Hubbell, and C.-S. Su. Poly (4-vinylimidazole) as nonviral gene carrier: in vitro and in vivo transfection. Acta Biomaterialia1:165–172 (2005).
E. S. Lee, H. J. Shin, K. Na, and Y. H. Bae. Poly (L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J. Control. Release90:363–374 (2003).
S. Patnaik, A. Aggarwal, A. Goel, M. Ganguli, N. Saini, Y. Singh, and K. C.Gupta. PEI-alginate nanocomposites as efficient in vitro gene transfection agents. J. Control. Release114:398–409 (2006).
M. Koping-Hoggard, I. Tubulekas, H. Guan, K. Edwards, M. Nilsson, K. M. Varum, and P. Artursson. Chitosan as a nonviral gene delivery system. Structure-property relationships and characteristics compared with polyethylenimine in vitro and after lung administration in vivo. Gene Ther.8:1108–1121 (2001).
Acknowledgements
The authors are thankful to Sophisticated Analytical Instrument Facility, Central Drug Research Institute, Lucknow, India and NMR Laboratory, Indian Institute of Technology, Delhi for NMR analysis. Authors (AP, RKK, SP and AS) gratefully acknowledge the Indian Council for Medical Research (ICMR), the Council of Scientific and Industrial Research (CSIR) and the University Grant Commission (UGC), respectively, for providing financial support.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Pathak, A., Aggarwal, A., Kurupati, R.K. et al. RETRACTED ARTICLE: Engineered Polyallylamine Nanoparticles for Efficient In Vitro Transfection. Pharm Res 24, 1427–1440 (2007). https://doi.org/10.1007/s11095-007-9259-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9259-7