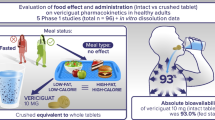
Abstract
Purpose
To examine the effect of common excipients such as sugars (sorbitol versus sucrose) on bioequivalence between pharmaceutical formulations, using ranitidine and metoprolol as model drugs.
Methods
Two single-dose, replicated, crossover studies were first conducted in healthy volunteers (N = 20 each) to compare the effect of 5 Gm of sorbitol and sucrose on bioequivalence of 150 mg ranitidine or 50 mg metoprolol in aqueous solution, followed by a single-dose, nonreplicated, crossover study (N = 24) to determine the threshold of sorbitol effect on bioequivalence of 150 mg ranitidine in solution.
Results
Ranitidine Cmax and AUC(0–∞) were decreased by ∼50% and 45%, respectively, in the presence of sorbitol versus sucrose. Similarly, sorbitol reduced metoprolol Cmax by 23% but had no significant effect on AUC(0–∞). An appreciable subject-by-formulation interaction was found for ranitidine Cmax and AUC(0–∞), as well as metoprolol Cmax. Sorbitol decreased the systemic exposure of ranitidine in a dose-dependent manner and affected bioequivalence at a level of 1.25 Gm or greater.
Conclusions
As exemplified by sorbitol, some common excipients have unexpected effect on bioavailability/bioequivalence, depending on the pharmacokinetic characteristics of the drug, as well as the type and amount of the excipient present in the formulation. More research is warranted to examine other ‘common’ excipients that may have unintended influence on bioavailability/bioequivalence.




Similar content being viewed by others
References
G. L. Amidon, H. Lennernas, V. P. Shah, and J. R. Crison. A theoretical basis for abiopharmaceutics drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12:413–420 (1995).
U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry: Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System, Office of Training and Communications, Division of Communications Management, Drug Information Branch, HFD-210, Rockville Maryland 20857, August 2000.
D. M. Woodcock, S. Jefferson, M. E. Linsenmeyer, P. J. Crowther, G. M. Chojnowski, B. Williams, and I. Bertoncello. Reversal of the multi-drug resistance phenotype with Cremophor EL, a common vehicle for water-insoluble vitamins and drugs. Cancer Res. 50:4199–4203 (1990).
A. R. Fassihi, R. Dowse, and S. S. D. Robertson. Influence of sorbitol solution on the bioavailability of theophylline. Int. J. Pharm. 72:175–178 (1991).
K. M. Koch, A. F. Parr, J. J. Tomlinson, E. P. Sandefer, G. A. Digenis, K. H. Donn, and J. R. Powell. Effect of sodium acid pyrophosphate on ranitidine bioavailability and gastrointestinal transit time. Pharm. Res. 10:1027–1030 (1993).
D. A. Adkin, S. S. Davis, R. A. Sparrow, P. D. Huckle, A. J. Philips, and I. R. Wilding. The effects of pharmaceutical excipients on small intestinal transit. Br. J. Clin. Pharmacol. 39:381–387 (1995).
P. P. Constantinides. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm. Res. 12:1561–1572 (1995).
D. A. Adkin, S. S. Davis, R. A. Sparrow, P. D. Huckle, and I. R. Wilding. The effect of mannitol on the oral bioavailability of cimetidine. J. Pharm. Sci. 84:1405–1409 (1995).
R. A. Rajewski and V. J. Stella. Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery. J. Pharm. Sci. 85:1142–1169 (1996).
L. Yu, A. Bridgers, J. Polli, A. Vickers, S. Long, A. Roy, R. Winnike, and M. Coffin. Vitamin E-TPGS increases absorption flux of an HIV protease inhibitor by enhancing its solubility and permeability. Pharm. Res. 16:1812–1817 (1999).
B. J. Aungst. Intestinal permeation enhancers. J. Pharm. Sci. 89:429–442 (2000).
A. Bernkop-Schnurch. Chitosan and its derivatives: potential excipients for peroral peptide delivery systems. Int. J. Pharm. 194:1–13 (2000).
A. Bernkop-Schnurch and C. E. Kast. Chemically modified chitosans as enzyme inhibitors. Adv. Drug Deliv. Rev. 52:127–137 (2001).
A. W. Basit, F. Podczeck, J. M. Newton, W. A. Waddington, P. J. Ell, and L. F. Lacey. Influence of polyethylene glycol 400 on the gastrointestinal absorption of ranitidine. Pharm. Res. 19:1368–1374 (2002).
M. Martin-Facklam, J. Burhenne, R. Ding, R. Fricker, G. Mikus, I. Walter-Sack, et al. Dose-dependent increase of saquinavir bioavailability by the pharmaceutic aid Cremorphor EL. Br. J. Clin. Pharmacol. 53:576–581 (2002).
Y. Tayrouz, R. Ding, J. Burhenne, K. D. Riedel, J. Weiss, T. Hoppe-Tichy, W. E. Haefeli, and G. Mikus. Pharmacokinetic and pharmaceutic interaction between digoxin and Cremophor RH40. Clin. Pharmacol. Ther. 73:397–405 (2003).
C. Wandel, R. Kim, and M. Stein. “Inactive” excipients such as Cremophor can affect in vivo drug disposition. Clin. Pharmacol. Ther. 73:394–396 (2003).
M.-L. Chen and L. J. Lesko. Individual bioequivalence revisited. Clin. Pharmacokinet. 40:701–706 (2001).
D. Farthing, K. L. R. Brouwer, I. Fakhry, and D. Sica. Solid-phase extraction and determination of ranitidine in human plasma by a high-performance liquid chromatographic method utilizing midbore chromatography. J. Chromatogr. B. 688:350–353 (1997).
B. Mistry, J. Leslie, and N. E. Eddington. A sensitive assay of metoprolol and its metabolite α-hydroxy metoprolol in human plasma and determination of dextromethorphan and its metabolite dextrorphan in urine with high performance liquid chromatography and fluorometric detection. J. Pharm. Biomed. Anal. 16:1041–1049 (1998).
D. J. Schuirmann. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J. Pharmacokinet. Biopharm. 15:657–680 (1987).
U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry: Statistical Approaches to Establishing Bioequivalence, Office of Training and Communications, Division of Communications Management, Drug Information Branch, HFD-210, Rockville Maryland 20857, January 2001.
M.-L. Chen, R. Patnaik, W. W. Hauck, D. J. Schuirmann, T. Hyslop, and R. L. Williams. An individual bioequivalence criterion: regulatory considerations. Stat. Med. 19:2821–2842 (2000).
W. W. Hauck, T. Hyslop, M.-L. Chen, R. N. Patnaik, D. J. Schuirmann, R. L. Williams, and FDA Population/Individual Bioequivalence Working Group. Subject-by-formulation interaction in bioequivalence: conceptual and statistical issues. Pharm. Res. 17:375–380 (2000).
A. N. Wick, M. C. Almen, and L. Joseph. Metabolism of sorbitol. J. Am. Pharm. Assoc. 40:542–544 (1951).
J. D. Cryboski. Diarrhea from dietetic candies. N. Engl. J. Med. 275:718 (1966).
J. S. Hyams. Chronic abdominal pain caused by sorbitol malabsorption. J. Pediatr. 100:772–773 (1982).
R. E. Hill and K. R. Kamath. “Pink” diarrhea. Med. J. Aust. 1:387–389 (1982).
N. K. Jain, D. B. Rosenberg, M. J. Ulahannan, M. J. Glasser, and C. S. Pitchumoni. Sorbitol intolerance in adults. Am. J. Gastroenterol. 80:678–681 (1985).
J. S. Hyams. Sorbitol intolerance: an unappreciated cause of functional gastrointestinal complaints. Gastroenterol. 84:30–33 (1983).
D. B. Rosenberg, N. K. Jain, M. J. Ulahannan, et al. Sorbitol intolerance in adults and its relationship to lactose intolerance. Gastroenterol. 86:1356 (1984).
N. K. Jain, V. P. Patel, and C. S. Pitchumoni. Sorbitol intolerance in adults: prevalence and pathogenesis on two continents. J. Clin. Gastroenterol. 9:317–319 (1987).
M. S. Badiga, N. K. Jain, C. Casanova, and C. S. Pitchumoni. Diarrhea in diabetics: the role of sorbitol. J. Am. Coll. Nutr. 9:578–582 (1990).
D. Mishkin, L. Sablauskas, M. Yalovsky, and S. Mishkin. Fructose and sorbitol malabsorption in ambulatory patients with functional dyspepsia: comparison with lactose maldigestion/malabsorption. Dig. Dis. Sci. 42:2591–2598 (1997).
M. M. Smith, M. Davis, F. I. Chasalow, and F. Lifshitz. Carbohydrate absorption from fruit juice in young children. Pediatrics 95:340–344 (1995).
T. Nobigrot, F. I. Chasalow, and F. Lifshitz. Carbohydrate absorption from one serving of fruit juice in young children: age and carbohydrate composition effects. J. Am. Coll. Nutr. 16:152–158 (1997).
M. Marvola, A. Reinikainen, M. L. Heliovaara, and A. Huikari. The effects of some sweetening agents and osmotic pressure on the intestinal absorption of sulfafurazole in the rat. J. Pharm. Pharmacol. 31:615–618 (1979).
M. F. Williams, G. E. Dukes, W. Heizer, Y.-H. Han, D. J. Hermann, T. Lampkin, and L. J. Hak. Influence of gastrointestinal site of drug delivery on the absorption characteristics of ranitidine. Pharm. Res. 9:1190–1194 (1992).
T. Grammatte, E. El. Desoky, and U. Klotz. Site-dependent small intestinal absorption of ranitidine. Eur. J. Clin. Pharmacol. 46:253–259 (1994).
D. J. Kazierad, K. D. Schlanz, and M. B. Bottorff. Beta blockers. In W. E. Evans, J. J. Schentag, and W. J. Jusko (eds.), Applied Pharmacokinetics — Principles of Therapeutic Drug Monitoring, 3rd ed, Applied Therapeutics, Vancouver, WA, 1995, pp. 24–31.
D. L. Bourdet, J. B. Pritchard, and D. R. Thakker. Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC22A2), and hOCT3 (SLC22A3). J. Pharmacol. Exp. Ther. 315:1288–1297 (2005).
U. Klotz and S. Walker. Biliary excretion of H2 receptor antagonists. Eur. J. Clin. Pharmacol. 29:91–92 (1990).
N. Takamatsu, L. S. Welage, Y. Hayashi, R. Yamamoto, J. L. Barnett, V. P. Shah, L. J. Lesko, C. Ramachandran, and G. L. Amidon. Variability in cimetidine absorption and plasma double peaks following oral administration in the fasted state in humans: correlation with antral gastric motility. Eur. J. Pharm. Biopharm. 53:37–47 (2002).
A. Minocha, E. P. Krenzelok, and D. A. Spyker. Dosage recommendations for activated charcoal–sorbitol treatment. Clin. Toxicol. 23:579–587 (1985).
M. J. Ellenhorn and D. G. Barceloux. Medical Toxicology Diagnosis and Treatment of Human Poisoning. Elsevier, New York, 1988, p. 9.
J. Glauser. Tricyclic antidepressant poisoning. Clevel. Clin. J. Med. 67:704–719 (2000).
American Academy of Clinical Toxicology and European Association of Poisons Centres and Clinical Toxicologists. Position paper: cathartics. J. Toxicol., Clin Toxicol. 42:243–253 (2004).
E. P. Krenzelok, R. Keller, and R. D. Stewart. Gastrointestinal transit times of cathartics combined with charcoal. Ann. Emerg. Med. 14:1152–1155 (1985).
M. Mayersohn, D. Perrier, and A. L. Picchioni. Evaluation of a charcoal–sorbitol mixture as an antidote for oral aspirin overdose. Clin. Toxicol. 11:561–567 (1977).
A. H. Al-Shareef, D. C. Buss, E. M. Allen, and P. A. Routledge. The effects of charcoal and sorbitol (alone and in combination) on plasma theophylline concentration after a sustained-release formulation. Human Exp. Toxicol. 9:179–182 (1990).
N. A. Minton and J. A. Henry. Prevention of drug absorption in simulated theophylline overdose. Clin. Toxicol. 33:43–49 (1995).
E. C. Scholtz, J. M. Jaffe, and J. L. Colazzi. Evaluation of five activated charcoal formulations for inhibition of aspirin absorption and palatability in man. Am. J. Hosp. Pharm. 35:1355–1359 (1978).
R. M. McNamara, C. K. Aaron, M. Gemborys, and S. Davidheiser. Sorbitol catharsis does not enhance efficacy of charcoal in a simulated acetaminophen overdose. Ann. Emerg. Med. 17:243–246 (1988).
C.-Y. Wu and L. Z. Benet. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics disposition classification system. Pharm. Res. 22:11–23 (2005).
M. Lindenberg, S. Kopp, and J. B. Dressman. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur. J. Pharm. Biopharm. 58:265–278 (2004).
Goodman and Gilman’s The Pharmacological Basis of Therapeutics. In J. G. Hardman and L. E. Limbird (eds.-in-chief), P. B. Molinoff and R. W. Ruddon (eds.), A. G. Gilman (consulting ed.), 9th ed. McGraw-Hill, New York, 1995, p. 1712.
R. E. Keller, R. A. Schwab, and E. P. Krenzelok. Contribution of sorbitol combined with activated charcoal in prevention of salicylate absorption. Ann. Emerg. Med. 19:654–656 (1990).
Digestion and absorption in the gastrointestinal tract. Chapter 65. In A. C. Guyton and J. E. Hall (eds.), Textbook of Medicinal Physiology, 9th ed, W.B. Saunders, Philadelphia, 1996, p. 834.
D. Kruger, R. Grossklaus, M. Herold, S. Lorenz, and L. Klingebiel. Gastrointestinal transit and digestibility of maltitol, sucrose and sorbitol in rats: a multicompartmental model and recovery study. Experientia 48:733–740 (1992).
H. A. Krebs. Some general considerations concerning the use of carbohydrates in parenteral nutrition. In I. D. A. Johnston (ed.), Advances in Parenteral Nutrition, MTP, Lancaster, 1978, pp. 23–28.
E. M. Hill, C. M. Flaitz, and G. R. Frost. Sweetener content of common pediatric oral liquid medications. Am. J. Hosp. Pharm. 45:135–142 (1988).
D. M. Lutomski, M. L. Gora, S. M. Wright, and J. E. Martin. Sorbitol content of selected oral liquids. Ann. Pharmacother. 27:269–274 (1993).
A. Kumar, R. D. Rawlings, and D. C. Beaman. The mystery ingredients: sweeteners, flavorings, dyes, and preservatives in analgesic/antipyretic, antihistamine/decongestant, cough and cold, antidiarrheal, and liquid theophylline preparations. Pediatrics 91:927–933 (1993).
R. E. Wrolstad and R. S. Shallenberger. Free sugars and sorbitol in fruits — A compilation from the literature. J. Assoc. Off. Anal. Chem. 64:91–103 (1981).
A. A. Moukarzel and M. T. Sabri. Gastric physiology and functions: effects of fruit juices. J. Am. Coll. Nutr. 15:18S–25S (1996).
R. A. Breitenbach. ‘Halloween diarrhea’ — An unexpected trick of sorbitol-containing candy. Postgrad. Med. 92:63–66 (1992).
Acknowledgments
This work was supported, in part, by a contract from the Food and Drug Administration to the University of Tennessee, Memphis, Tennessee. The authors would like to thank Lawrence Lesko, Rabindra Patnaik and Lawrence Yu for their helpful discussion on the related topics during the early phase of this investigation.
Author information
Authors and Affiliations
Corresponding author
Additional information
The opinions expressed in this article are those of the authors and do not necessarily represent the views or policies of the Food and Drug Administration.
Rights and permissions
About this article
Cite this article
Chen, ML., Straughn, A.B., Sadrieh, N. et al. A Modern View of Excipient Effects on Bioequivalence: Case Study of Sorbitol. Pharm Res 24, 73–80 (2007). https://doi.org/10.1007/s11095-006-9120-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9120-4