Abstract
Abstract
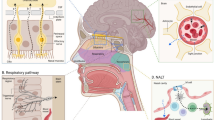
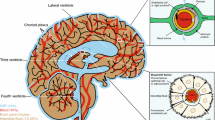
With application of molecular biology techniques, there has been rapid progress in understanding how many drugs and micronutrients (e.g., vitamins) are transferred across the choroid plexus (CP), the main transport locus of the blood–cerebrospinal fluid (CSF) barrier, and the renal tubular epithelial cells. In many cases, these molecules are transported by separate, specific carriers or receptors on the apical and/or basal side of the CP or renal epithelial cells. This commentary focuses on four micronutrient transport systems in CP (ascorbic acid, folate, inositol, and riboflavin), all of which have been recently cloned, expressed and for which knockout mice models were developed and transporter localization studies performed. Also reviewed is the recently cloned uric acid transport system in human kidney in which there exists a human “knockout” model. The implications of these transport systems for drug therapy of central nervous system and renal disorders are discussed, especially with regard to methods to circumvent the blood–brain and blood–CSF barriers to deliver drugs to the brain.

Similar content being viewed by others
References
H. Davson and M. B. Segal. Physiology of the CSF and Blood–Brain Barriers, CRC, Boca Raton, 1996.
R. Spector and C. E. Johanson. The mammalian choroid plexus: structure, development and function. Sci.Am. 261:68–74 (1989).
R. Spector. Drug transport in the mammalian central nervous system: multiple complex systems. Pharmacology60:58–73 (2000).
D. E. Smith, C. E. Johanson, and R. F. Keep. Peptide and peptide analog transport systems at the blood–CSF barrier. In Y. Sugiyama and J-F. Ghersi-Egea (eds.), Drug Transfer in the Choroid Plexus. Multiplicities and Substrate Specificities of Transporters, Adv. Drug Deliv. Rev., 56:1765–1791 (2004).
C. E. Johanson. The choroid plexus–CSF nexus: gateway to the brain. In P. M. Conn (ed.), Neuroscience in Medicine, 2nd ed., Humana, Totowa, New Jersey, 2003, pp. 165–195.
R. Spector. Megavitamin therapy and the central nervous system. In M. H. Briggs (ed.), Vitamins in Human Biology andMedicine, CRC, Boca Raton, Florida, 1981, pp. 138–156.
R. Spector. Vitamin homeostasis in the central nervous system. N. Engl. J. Med. 296:1393–1398 (1977) (Seminar in Medicine).
K. M. Giacomini and Y. Sugiyama. Membrane transporters and drug response. In L. L. Brunton, J. S. Lazo, and K. L. Parker (eds.), Goodman and Gilman's The Pharmacological Basis of Therapeutics, McGraw Hill, New York, 2005, pp. 41–70.
R. Spector. Micronutrient homeostasis in mammalian brain and cerebrospinal fluid. J. Neurochem. 53:1667–1674 (1989).
Y. Nagata, H. Kusuhara, H. Endou, and Y. Sugiyama. Expression and functional characterization of rat organic anion transporter 3 (rOAT 3) in the choroid plexus. Mol. Pharm. 61:982–988 (2002).
W. M. Pardridge, Introduction to the Blood–Brain Barrier. Cambridge University Press, UK, 1998.
J. B. Pritchard and D. S. Miller. Expression systems for cloned xenobiotic transporters. Toxicol. Appl. Pharmacol. 204:256–262 (2005).
M. E. Rice. Ascorbate regulation and its neuroprotective role in brain. TrendsNeurosci. 23:209–216 (2000).
L. Hammarstrom. Autoradiographic studies on the distribution of 14C-labelled ascorbic acid and dehydroascorbic acid. Acta Physiol. Scand.70(suppl. 289):1–79 (1966).
R. Spector. Penetration of ascorbic acid from cerebrospinal fluid into brain. Exp. Neurol. 72:645–653 (1981).
A. Hakvoort, M. Haselbach, and H. J. Galla. Active transport properties of porcine choroid plexus cells in culture. Brain Res. 795:247–256 (1998).
S. Angelow, P. Zeni, and H. J. Galla. Usefulness and limitation of primary cultured porcine choroid plexus epithelial cells as an in vitro model to study drug transport at the blood–CSF barrier. Adv. Drug Del. Rev. 56:1859–1873 (2004).
R. Spector and A. V. Lorenzo. The specificity of ascorbic acid transport system of the central nervous system. Am. J. Physiol. 226:1468–1473 (1974).
D. K. C. Lam and P. M. Daniel. The influx of ascorbic acid into the rat's brain. Quart. J. Exp. Physiol. 71:483–489 (1986).
D. B. Agus, S. S. Bambhir, W. M. Pardridge, et al. Vitamin C crosses the blood–brain barrier in the oxidized form through the glucose transporters. J. Clin. Invest. 100:2842–2848 (1997).
H. Tsubaguchi, T. Tokui, B. Mackenzie, et al. A family of mammalian Na+-dependent l-ascorbic acid transporters. Nature399:70–75 (1999).
A. Astuya, T. Caprile, M. Castro, et al. Vitamin C uptake and recycling among normal and tumor cells from the central nervous system. J. Neurosci. Res. 79:146–156 (2005).
M. D. L. A. Garcia, K. Salazar, C. Millan, et al. Sodium vitamin C cotransporter SVCT 2 is expressed in hypothalamic glial cells. Glia50:32–47 (2005).
S. Sotiriou, S. Gispert, J. Cheung, et al. Ascorbic acid slc 23 a1 is essential for vitamin C transport into brain and for perinatal survival. Nat. Med. 8:514–517 (2002).
J. Hung, D. B. Agus, C. J. Winfree, et al. Dehydorascorbic acid, a blood–brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. PNAS98:11720–11724 (2001).
R. Spector and A. V. Lorenzo. Folate transport by the choroid plexus in vitro. Science187:540–542 (1975).
R. Spector and A. V. Lorenzo. Folate transport in the central nervous system. Am. J. Physiol.229:777–782 (1975).
S. A. Suleiman and R. Spector. Purification and characterization of a folate binding protein from porcine choroid plexus. Arch. Biochem. Biophys.208:87–94 (1981).
Y. Wang, R. Zhao, R. G. Russel, and I. D. Goldman. Localization of the murine reduced folate carrier as assessed by immunohistochemical analysis. Biochim. Biophys. Acta1513:49–54 (2001).
V. T. Ramaekers, S. I. Hansen, J. Holm, et al. Reduced folate transport to the CNS in female Rett patients. Neurology61:506–515 (2003).
R. Spector. Development of the vitamin transport system in choroid plexus and brain. Neurochemistry33:1317–1319 (1979).
B. A. Kamen and A. K. Smith. A review of folate receptor alpha and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv. Drug Del. Rev.56:1085–1097 (2004).
D. Wa and W. M. Pardridge. Blood–brain transport of reduced folic acid. Pharm. Res.16:415–419 (1999).
V. T. Ramaekers and N. Blau. Cerebral folate deficiency. Dev. Med. Child Neurol.46:843–851 (2004).
J. A. Piedrahita, B. Oetama, G. D. Bennett, et al. Mice lacking the folic-acid binding protein Folbp1 are defective in early embryonic development. Nat. Genet.23:228–232 (1999).
V. T. Ramaekers, S. P. Rothenberg, J. M. Sequeira, et al. Auto antibodies to folate receptors in the cerebral folate deficiency syndrome. N. Engl. J. Med.352:1985–1991 (2005).
S. Xiao, D. K. Hansen, E. T. M. Horsley, et al. Maternal folate deficiency results in selective upregulation of folate receptors and heterogenous nuclear ribonucleoprotein-E1 associated with multiple subtle aberrations in fetal tissues. Birth Defects Res. (Part A): Clin. and Mol Teratol.73:6–28 (2005).
S. K. Fischer, J. E. Novak, and B. W. Agranoff. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J. Neurochem.82:736–754 (2002).
R. Spector and A. V. Lorenzo. Myo-inosital transport in central nervous system. Am. J. Physiol.228:1510–1518 (1975).
R. Spector and A. V. Lorenzo. The origin of myo-inositol in brain, cerebrospinal fluid and choroid plexus. J. Neurochem.25:353–354 (1975).
R. Spector. The specificity and sulfhydryl sensitivity of the inositol transport system of the central nervous system. J. Neurochem.27:229–236 (1976).
R. Spector. Myo-inositol transport through the blood–brain barrier. Neurochem. Res.13:785–787 (1988).
R. Spector. Inositol accumulation by brain slices in vitro. J. Neurochem.27:1273–1276 (1976).
I. Inoue, S. Shimoda, Y. Minami, et al. Cellular localization of Na+/myo-inositol 1 co-transporter in RNA in the rat brain. NeuroReport7:1195–1198 (1996).
M. J. Coady, B Wallendorff, D. G. Gagnon, and J. Y. Lapointe. Identification of a novel Na+/myo-inositol cotransporter. J. Biol. Chem.277:35219–35224 (2002).
Y. Minami, K. Inoue, S. Shimada, et al. Rapid and transient upregulation of Na+/myo-inositol cotransporter transcription in the brain of acute hypernatremic rats. Mol. Brain Res.40:64–70 (1996).
G. T. Berry, S. Wu, R. Buccafusca, et al. Loss of murine Na+/myo-inositol cotransporter leads to brain myo-inositol depletion and central apnea. J. Biol. Chem.278:18297–18302 (2003).
J. F. L. Chau, M. K. Lee, J. W. Law, et al. Sodium myo-inositol cotransporter-1 is essential for the development and function of the peripheral nerves. FASEB J. 19:1887–1889 (2005).
C. M. Moore, J. L. Breeze, T. J. Kukes, et al. Effects of myo-inositol ingestion on human brain myo-inositol levels: a proton magnetic resonance spectroscopic study. Biol. Psychiatry45:1197–1202 (1999).
G. T. Barry, R. Buccafusca, J. J. Greer, and E. Eccleston. Phosphoinositide deficiency due to inositol depletion is not a mechanism of lithium action in brain. Mol. Genet. Metab. 82:87–92 (2004).
J. R. Alack. Inositol monophosphate inhibitors—lithium mimetics? Med. Res. Rev. 17:215–224 (1997).
S. E. Bresler, V. M. Bresler, E. N. Kazbekov, A. A. Nikitorov, and N. N. Vasilieva. On the active transport of organic acids fluorescein in the choroid plexus of the rabbit. Biochim. Biophys. Acta. 550:110–119 (1979).
R. Spector. Riboflavin transport in the central nervous system: characterization and effects of drugs. J. Clin. Invest. 66:821–831 (1980).
R. Spector and B. Boose. Active transport of riboflavin by the isolated choroid plexus in vitro. J. Biol. Chem. 254:10286–10289 (1979).
R. Spector. Riboflavin homeostasis in the central nervous system. J. Neurochem. 35:202–209 (1980).
R. Spector. Riboflavin accumulation by rabbit brain slices in vitro. J. Neurochem. 34:1768–1771 (1980).
R. Spector. Lumiflavin and lumichrome transport in the central nervous system. J. Neurochem. 36:1186–1191 (1981).
R. Spector and A. V. Lorenzo. Inhibition of penicillin transport from the cerebrospinal fluid after intracranial inoculation of bacteria. J. Clin. Invest. 54:316–325 (1974).
D. H. Sweet, D. S. Miller, J. B. Pritchard, Y Fiyuvare, D. R. Brier, and S. K. Nigam. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat 3 slc22a8) knockout mice. J. Biol. Chem. 277:26934–26943 (2002).
D. Sykes, D. H. Sweet, S. Lowes, S. K. Nigam, J. B. Pritchard, and D. S. Miller. Organic anion transport in choroid plexus from wild-type and organic anion transporter 3 (slc22a8)-null mice. Am. J. Physiol. 286:F972–F978 (2004).
R. Kikuchi, H. Kusuhara, D. Sugiyama, and Y. Sugiyama. Contribution of organic anion transporter 3 (slc22a8) to the elimination of p-aminohippuric acid and benzylpenicillin across the blood–brain barrier. J. Pharmacol. Exp. Ther. 306:51–58 (2003).
C. M. Breen, D. B. Sykes, G. Fricker, and D. S. Miller. Confocal imaging of organic anion transport in intact rat choroid plexus. Am. J. Physiol. 282:F877–F885 (2002).
S. M. Ocheltree, H. Shen, Y. Hu, J. Xiang, R. F. Keep, and D. E. Smith. Mechanisms of cefadroxil uptake in choroid plexus: studies in wild-type and PEPT 2 knock-out mice. J. Pharmacol. Exp. Ther. 308:462–467 (2004).
D. S. Miller. Confocal imaging of xenobiotic transport across the choroid plexus. Adv. Drug Del. Rev. 56:1811–1824 (2004).
W. J. Jusko and G. Levy. Absorption, protein binding and elimination of riboflavin. In Rivlin (ed.), Riboflavin, Plenum, New York, 1975, pp. 100–152.
R. Spector. Riboflavin transport by rabbit kidney slices: characterization and relation of cyclic organic acid transport. J. Pharmacol. Exp. Ther. 221:394–398 (1982).
A. Enomoto, H. Kimura, A. Chairougdue, et al. Molecular identification of a renal-urate exchanger that regulates blood urate levels. Nature417:447–451 (2002).
K. Ichida, M. Hosoyamada, I. Hisatome, et al. Clinical and molecular analysis of patients with renal hypouricemia in Japan—influence of URAT 1 gene on urinary urate excretion. J. Am. Soc. Nephrol. 15:164–173 (2004).
D. Kang, L. Han, X. Ouyang, A. Kahn, et al. Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. Am. J. Nephrol. 25:425–433 (2005).
C. Johanson, J. Duncan, E. Stopa, and A. Baird. Enhanced prospects for drug delivery and brain targeting by the choroid plexus–CSF route. Pharm. Res. 22:1011–1037 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spector, R., Johanson, C. Micronutrient and Urate Transport in Choroid Plexus and Kidney: Implications for Drug Therapy. Pharm Res 23, 2515–2524 (2006). https://doi.org/10.1007/s11095-006-9091-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9091-5
Key words
- ascorbic acid
- blood–brain barrier
- blood–cerebrospinal fluid (CSF) barrier
- choroid plexus epithelium
- folate
- folate receptor (FRα)
- inositol
- myo-inositol cotransporter (SMIT 1)
- organic acid transporter 3 (OAT 3)
- penicillin
- riboflavin
- sodium ascorbate cotransporter (SVCT 2)
- uric acid transporter (URAT 1)
- vitamin homeostasis