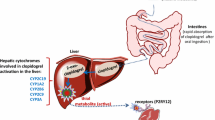
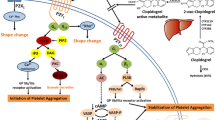
Abstract
Antiplatelet therapy provided pivotal advances in the treatment of cardiovascular disease. Aspirin and thienopyridine, clopidogrel, is currently the treatment of choice in acute coronary syndromes and the prevention of thrombosis after coronary stent implantation. Despite the efficacy of this dual antiplatelet therapy in reduction of adverse coronary events in patients with acute coronary syndromes, complications persist in a subgroup of these patients. Emerging causes of aspirin and clopidogrel resistance may translate to increase risk for recurrent myocardial infarction, stroke, or cardiac related mortality. However, the mechanism of antiplatelet drug resistance remains incompletely characterized, and a sensitive and specific assay of aspirin and clopidogrel effect that reliably predicts treatment failure has not emerged. To date, evidence supporting antiplatelet drug resistance are pharmacokinetic response variability, drug-drug interaction through competitive inhibition a specific enzymatic pathway, genetic variability, and variability in the induction of enzymatic pathway in metabolic activation of prodrugs, like clopidogrel. Further investigation or guidelines are needed to optimize antiplatelet treatment strategies to identify and treat patients resistant to aspirin and/or clopidogrel.











Similar content being viewed by others
References
P. A. Gurbel, K. P., Bliden, K. M. Hayes, et al. Platelet activation in myocardial ischemic syndromes. Expert Rev. Cardiovasc. Ther. 2:535–545 (2004).
A. P. Selwyn. Prothrombotic and antithrombotic pathways in acute coronary syndromes. Am. J. Cardiol. 91:3H–11H (2003).
K. H. Mak, G. Belli, S. G. Ellis, et al. Subacute stent thrombosis: evolving issues and current concepts. J. Am. Coll. Cardiol. 27:494–503 (1996).
A. Budaj, S. Yusuf, S. R. Mehta, et al. Benefit of clopidogrel in patients with acute coronary syndromes without ST-segment elevation in various risk groups. Circulation 106:1622–1626 (2002).
S. Yusuf, F. Zhao, S. R. Mehta, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 345:494–502 (2001).
D. L. Bhatt, M. E. Bertrand, P. B. Berger, et al. Meta-analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stenting. J. Am. Coll. Cardiol. 39:9–14 (2002).
Antiplatelet Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324:71–86 (2002).
B. Rocca, and C. Patrono. Determinants of the interindividual variability in response to antiplatelet drugs. J. Thromb. Haemost. 3:1597–1602 (2005).
S. R. Steinhubl, R. Charnigo, and D. J. Moliterno. Resistance to antiplatelet resistance is it justified? J. Am. Coll. Cardiol. 45:1757–1758 (2005).
C. P. Martin, and R. L. Talbert. Aspirin resistance: an evaluation of current evidence and measurement methods. Pharmacotherapy 25:942–953 (2005).
T. A. Nguyen, J. G. Diodati, and C. Pharand. Resistance to clopidogrel: a review of the evidence. J. Am. Coll. Cardiol. 45:1157–1164 (2005).
B. Stein, V. Fuster, D. H. Israel, et al. Platelet inhibitor agents in cardiovascular disease: an update. J. Am. Coll. Cardiol. 14:813–836 (1989).
A. I. Schafer. Antiplatelet therapy. Am. J. Med. 101:199–209 (1996).
T. Nakamura, J. Kambayashi, M. Okuma, et al. Activation of the GP IIb–IIIa complex induced by platelet adhesion to collagen is mediated by both alpha2beta1 integrin and GP VI. J. Biol. Chem. 274:11897–11903 (1999).
A. Schafer, N. M. Ali, and G. N. Levine. Hemostasis, thrombosis, fibrinolysis, and cardiovascular disease. In E. Braunwald, D. P. Zipes, and P. Libby. (eds.), 6th ed., Saunders, Philadelphia, 2001, pp. 2099–2132.
R. J., Shebuski, and K. S. Kilgore. Role of inflammatory mediators in thrombogenesis. J. Pharmacol. Exp. Ther. 300:729–735 (2002).
H. Loppnow, R. Bil, S. Hirt, et al. Platelet-derived interleukin-1 induces cytokine production, but not proliferation of human vascular smooth muscle cells. Blood 91:134–141 (1998).
F. Marti, E. Bertran, M. Llucia, et al. Platelet factor 4 induces human natural killer cells to synthesize and release interleukin-8. J. Leukoc. Biol. 72:590–597 (2002).
P. Patrignani, P. Filabozzi, and C. Patrono. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J. Clin. Invest. 69:1366–1372 (1982).
W. M. Samara, K. P. Bliden, U. S. Tantry, et al. The difference between clopidogrel responsiveness and posttreatment platelet reactivity. Thromb. Res. 115:89–94 (2005).
P. A., Gurbel, K. P. Bliden, K. M. Hayes, et al. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J. Am. Coll. Cardiol. 45:1392–1396 (2005).
P. A. Gurbel, W. M. Samara, and K. P. Bliden. Failure of clopidogrel to reduce platelet reactivity and activation following standard dosing in elective stenting: implications for thrombotic events and restenosis. Platelets 15:95–99 (2004).
N. S. Nicholson, S. G. Panzer-Knodle, N. F. Haas, et al. Assessment of platelet function assays. Am. Heart J. 135:S170–S178 (1998).
M. E. McKenzie, P. A. Gurbel, D. J. Levine, et al. Clinical utility of available methods for determining platelet function. Cardiology 92:240–247 (1999).
E. F. Mammen, R. S. Alshameeri, and P. C. Comp. Preliminary data from a field trial of the PFA-100 system. Semin. Thromb. Hemost. 21(Suppl 2):113–121 (1995).
E. F. Mammen, P. C. Comp, R. Gosselin, et al. PFA-100 system: a new method for assessment of platelet dysfunction. Semin. Thromb. Hemost. 24:195–202 (1998).
K. Kottke-Marchant, J. B. Powers, L. Brooks, et al. The effect of antiplatelet drugs, heparin, and preanalytical variables on platelet function detected by the platelet function analyzer (PFA-100). Clin. Appl. Thromb. Hemost. 5:122–130 (1999).
B. S. Coller, D. Lang, and L. E. Scudder. Rapid and simple platelet function assay to assess glycoprotein IIb/IIIa receptor blockade. Circulation 95:860–867 (1997).
R. M. Craft, J. J. Chavez, C. C. Snider, et al. Comparison of modified Thrombelastograph and Plateletworks whole blood assays to optical platelet aggregation for monitoring reversal of clopidogrel inhibition in elective surgery patients. J. Lab. Clin. Med. 145:309–315 (2005).
U. S. Tantry, K. P. Bliden, and P. A. Gurbel. Overestimation of platelet aspirin resistance detection by thrombelastograph platelet mapping and validation by conventional aggregometry using arachidonic acid stimulation. J. Am. Coll. Cardiol. 46:1705–1709 (2005).
J. W. Eikelboom, J. Hirsh, J. I. Weitz, et al. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation 105:1650–1655 (2002).
M. J., McQueen, E. Lonn, H. C. Gerstein, et al. The HOPE (Heart Outcomes Prevention Evaluation) Study and its consequences. Scand J. Clin. Lab. Invest. Suppl. 240:143–156 (2005).
M. Cattaneo. Aspirin and clopidogrel: efficacy, safety, and the issue of drug resistance. Arterioscler. Thromb. Vasc. Biol. 24:1980–1987 (2004).
P. A. Gum, K. Kottke-Marchant, P. A. Welsh, et al. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J. Am. Coll. Cardiol. 41:961–965 (2003).
W. H. Chen, P. Y. Lee, W. Ng, et al. Aspirin resistance is associated with a high incidence of myonecrosis after non-urgent percutaneous coronary intervention despite clopidogrel pretreatment. J. Am. Coll. Cardiol. 43:1122–1126 (2004).
K. Grundmann, K. Jaschonek, B. Kleine, et al. Aspirin non-responder status in patients with recurrent cerebral ischemic attacks. J. Neurol. 250:63–66 (2003).
A. D. Michelson, M. Cattaneo, J. W. Eikelboom, et al. Aspirin resistance: position paper of the Working Group on Aspirin Resistance. J. Thromb. Haemost. 3:1309–1311 (2005).
K. H. Grotemeyer, H. W. Scharafinski, and I. W. Husstedt. Two-year follow-up of aspirin responder and aspirin non responder. A pilot-study including 180 post-stroke patients. Thromb. Res. 71:397–403 (1993).
J. C. Wang, D. Aucoin-Barry, D. Manuelian, et al. Incidence of aspirin nonresponsiveness using the Ultegra Rapid Platelet Function Assay—ASA. Am. J. Cardiol. 92:1492–1494 (2003).
K. Andersen, M. Hurlen, H. Arnesen, et al. Aspirin non-responsiveness as measured by PFA-100 in patients with coronary artery disease. Thromb. Res. 108:37–42 (2002).
K. A. Schwartz, D. E. Schwartz, K. Ghosheh, et al. Compliance as a critical consideration in patients who appear to be resistant to aspirin after healing of myocardial infarction. Am. J. Cardiol. 95:973–975 (2005).
A. Bruno, J. P. McConnell, S. N. Cohen, et al. Serial urinary 11-dehydrothromboxane B2, aspirin dose, and vascular events in blacks after recent cerebral infarction. Stroke 35:727–730 (2004).
F. Cipollone, G. Ciabattoni, P. Patrignani, et al. Oxidant stress and aspirin-insensitive thromboxane biosynthesis in severe unstable angina. Circulation 102:1007–1013 (2000).
J. A. Cambria-Kiely, and P. J. Gandhi. Possible mechanisms of aspirin resistance. J. Thromb. Thrombolysis 13:49–56 (2002).
J. A. Cambria-Kiely, and P. J. Gandhi. Aspirin resistance and genetic polymorphisms. J. Thromb. Thrombolysis 14:51–58 (2002).
M. K. Halushka, L. P. Walker, and P. V. Halushka. Genetic variation in cyclooxygenase 1: effects on response to aspirin. Clin. Pharmacol. Ther. 73:122–130 (2003).
E. Ferrari, M. Benhamou, P. Cerboni, et al. Coronary syndromes following aspirin withdrawal: a special risk for late stent thrombosis. J. Am. Coll. Cardiol. 45:456–459 (2005).
M. Stillings, I. Havlik, M. Chetty, et al. Comparison of the pharmacokinetic profiles of soluble aspirin and solid paracetamol tablets in fed and fasted volunteers. Curr. Med. Res. Opin. 16:115–124 (2000).
P. J. Mason, A. K. Jacobs, and J. E. Freedman. Aspirin resistance and atherothrombotic disease. J. Am. Coll. Cardiol. 46:986–993 (2005).
M. Hurlen, I. Seljeflot, and H. Arnesen. Increased platelet aggregability during exercise in patients with previous myocardial infarction. Lack of inhibition by aspirin. Thromb. Res. 99:487–494 (2000).
T. Kawasaki, Y. Ozeki, T. Igawa, et al. Increased platelet sensitivity to collagen in individuals resistant to low-dose aspirin. Stroke 31:591–595 (2000).
E. H. Awtry, and J. Loscalzo. Aspirin. Circulation 101:1206–1218 (2000).
F. Catella-Lawson, M. P. Reilly, S. C. Kapoor, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N. Engl. J. Med. 345:1809–1817 (2001).
T. Kurth, R. J. Glynn, A. M. Walker, et al. Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal antiinflammatory drugs. Circulation 108:1191–1195 (2003).
T. M. MacDonald, and L. Wei. Effect of ibuprofen on cardioprotective effect of aspirin. Lancet 361:573–574 (2003).
S. E. Kimmel, J. A. Berlin, M. Reilly, et al. The effects of nonselective non-aspirin non-steroidal anti-inflammatory medications on the risk of nonfatal myocardial infarction and their interaction with aspirin. J. Am. Coll. Cardiol. 43:985–990 (2004).
D. Mukherjee, S. E. Nissen, and E. J. Topol. Risk of cardiovascular events associated with selective COX-2 inhibitors. Jama 286:954–959 (2001).
D. Mukherjee, S. E. Nissen, and E. J. Topol. Cox-2 inhibitors and cardiovascular risk: we defend our data and suggest caution. Clevel. Clin. J. Med. 68:963–964 (2001).
P. J. Newman, R. S. Derbes, and R. H. Aster. The human platelet alloantigens, PlA1 and PlA2, are associated with a leucine33/proline33 amino acid polymorphism in membrane glycoprotein IIIa, and are distinguishable by DNA typing. J. Clin. Invest. 83:1778–1781 (1989).
A. D. Michelson, M. I. Furman, P. Goldschmidt-Clermont, et al. Platelet GP IIIa Pl(A) polymorphisms display different sensitivities to agonists. Circulation 101:1013–1018 (2000).
A. H. Goodall, N. Curzen, M. Panesar, et al. Increased binding of fibrinogen to glycoprotein IIIa-proline33 (HPA-1b, PlA2, Zwb) positive platelets in patients with cardiovascular disease. Eur. Heart J. 20:742–747 (1999).
D. Burr, H. Doss, G. E. Cooke, et al. A meta-analysis of studies on the association of the platelet PlA polymorphism of glycoprotein IIIa and risk of coronary heart disease. Stat. Med. 22:1741–1760 (2003).
A. Undas, K. Brummel, J. Musial, et al. Pl(A2) polymorphism of beta(3) integrins is associated with enhanced thrombin generation and impaired antithrombotic action of aspirin at the site of microvascular injury. Circulation 104:2666–2672 (2001).
F. Cipollone, B. Rocca, and C. Patrono. Cyclooxygenase-2 expression and inhibition in atherothrombosis. Arterioscler. Thromb. Vasc. Biol. 24:246–255 (2004).
J. Maclouf, G. Folco, and C. Patrono. Eicosanoids and iso-eicosanoids: constitutive, inducible and transcellular biosynthesis in vascular disease. Thromb. Haemost. 79:691–705 (1998).
W. C. Lau, L. A. Waskell, P. B. Watkins, et al. Atorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation: a new drug–drug interaction. Circulation 107:32–37 (2003).
P. Savi, J. M. Pereillo, M. F. Uzabiaga, et al. Identification and biological activity of the active metabolite of clopidogrel. Thromb. Haemost. 84:891–896 (2000).
R. T. Dorsam, and S. P. Kunapuli. Central role of the P2Y12 receptor in platelet activation. J. Clin. Invest. 113:340–345 (2004).
J. A. Remijn, Y. P. Wu, E. H. Jeninga, et al. Role of ADP receptor P2Y(12) in platelet adhesion and thrombus formation in flowing blood. Arterioscler. Thromb. Vasc. Biol. 22:686–691 (2002).
C. Leon, M. Alex, A. Klocke, et al. Platelet ADP receptors contribute to the initiation of intravascular coagulation. Blood 103:594–600 (2004).
M. Gawaz. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc. Res. 61:498–511 (2004).
E. F. Plow, and T. Byzova. The biology of glycoprotein IIb–IIIa. Coron. Artery Dis. 10:547–551 (1999).
M. G. Rolf, C. A. Brearley, and M. P. Mahaut-Smith. Platelet shape change evoked by selective activation of P2X1 purinoceptors with alpha,beta-methylene ATP. Thromb. Haemost. 85:303–308 (2001).
C. Vial, S. J. Pitt, J. Roberts, et al. Lack of evidence for functional ADP-activated human P2X1 receptors supports a role for ATP during hemostasis and thrombosis. Blood 102:3646–3651 (2003).
C. Oury, M. J. Kuijpers, E. Toth-Zsamboki, et al. Overexpression of the platelet P2X1 ion channel in transgenic mice generates a novel prothrombotic phenotype. Blood 101:3969–3976 (2003).
P. B. Conley, and S. M. Delaney. Scientific and therapeutic insights into the role of the platelet P2Y12 receptor in thrombosis. Curr. Opin. Hematol. 10:333–338 (2003).
P. A. Gurbel, W. C. Lau, K. P. Bliden, et al. Clopidogrel resistance: implications for coronary stenting. Curr. Pharm. Des. 12:1261–1269 (2006).
D. Woulfe, H. Jiang, R. Mortensen, et al. Activation of Rap1B by G(i) family members in platelets. J. Biol. Chem. 277:23382–23390 (2002).
P. Lova, S. Paganini, F. Sinigaglia, et al. A Gi-dependent pathway is required for activation of the small GTPase Rap1B in human platelets. J. Biol. Chem. 277:12009–12015 (2002).
J. Geiger, J. Brich, P. Honig-Liedl, et al. Specific impairment of human platelet P2Y(AC) ADP receptor-mediated signaling by the antiplatelet drug clopidogrel. Arterioscler. Thromb. Vasc. Biol. 19:2007–2011 (1999).
P. Barragan, J. L. Bouvier, P. O. Roquebert, et al. Resistance to thienopyridines: clinical detection of coronary stent thrombosis by monitoring of vasodilator-stimulated phosphoprotein phosphorylation. Catheter. Cardiovasc. Interv. 59:295–302 (2003).
S. R. Steinhubl, P. B. Berger, J. T. Mann, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 288:2411–2420 (2002).
I. Muller, F. Besta, C. Schulz, et al. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb. Haemost. 89:783–787 (2003).
P. A. Gurbel, C. C. Cummings, C. R. Bell, et al. Onset and extent of platelet inhibition by clopidogrel loading in patients undergoing elective coronary stenting: the Plavix Reduction Of New Thrombus Occurrence (PRONTO) trial. Am. Heart J. 145:239–247 (2003).
D. E. Cutlip, D. S. Baim, K. K. Ho, et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation 103:1967–1971 (2001).
P. A. Gurbel, K. P. Bliden, B. L. Hiatt, et al. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 107:2908–2913 (2003).
D. R. Nelson, L. Koymans, T. Kamataki, et al. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics 6:1–42 (1996).
P. Savi, J. Combalbert, C. Gaich, et al. The antiaggregating activity of clopidogrel is due to a metabolic activation by the hepatic cytochrome P450-1A. Thromb. Haemost. 72:313–317 (1994).
M. Hasegawa, A. Sugidachi, T. Ogawa, et al. Stereoselective inhibition of human platelet aggregation by R-138727, the active metabolite of CS-747 (prasugrel, LY640315), a novel P2Y12 receptor inhibitor. Thromb. Haemost. 94:593–598 (2005).
M. Kazui, T. Ishizuka, N. Yamamura, et al. Mechanism of production of pharmacologically active metabolites of CS-747, a new pro-drug ADP-receptor antagonist. Thromb. Haemost. (sup):P1916 (2001).
W. C. Lau, L. A. Waskell, D. Carville, et al. The antiplatelet activity of clopidogrel is inhibited by atorvastatin but not by pravastatin. Circulation 102:2086 (2000) (abstract).
W. Jacobsen, B. Kuhn, A. Soldner, et al. Lactonization is the critical first step in the disposition of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor atorvastatin. Drug Metab. Dispos. 28:1369–1378 (2000).
T. A. Clarke, and L. A. Waskell. The metabolism of clopidogrel is catalyzed by human cytochrome P450 3A and is inhibited by atorvastatin. Drug Metab. Dispos. 31:53–59 (2003).
W. C. Lau, L. A. Waskell, P. Watkins, et al. The effect of drugs which are known inducers and inhibitors of human cytochrome P450 3A on the platelet inhibitory activity of clopidogrel. J. Am. Coll. Cardiol. 39:235A (2002).
C. Handschin, and U. A. Meyer. Induction of drug metabolism: the role of nuclear receptors. Pharmacol. Rev. 55:649–673 (2003).
H. M. Bolt. Rifampicin, a keystone inducer of drug metabolism: from Herbert Remmer's pioneering ideas to modern concepts. Drug Metab. Rev. 36:497–509 (2004).
J. M. Rae, M. D. Johnson, M. E. Lippman, et al. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. J. Pharmacol. Exp. Ther. 299:849–857 (2001).
B. Goodwin, E. Hodgson, and C. Liddle. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol. Pharmacol. 56:1329–1339 (1999).
W. C. Lau, P. A. Gurbel, P. B. Watkins, et al. Contribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistance. Circulation 109:166–171 (2004).
S. D. Wiviott, and E. M Antman. Clopidogrel resistance: a new chapter in a fast-moving story. Circulation 109:3064–3067 (2004).
P. A. Gurbel, K. P. Bliden, W. Samara, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J. Am. Coll. Cardiol. 46:1827–1832 (2005).
S. Matetzky, B. Shenkman, V. Guetta, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 109:3171–3175 (2004).
P. A. Gurbel, A. I. Malinin, K. P. Callahan, et al. Effect of loading with clopidogrel at the time of coronary stenting on platelet aggregation and glycoprotein IIb/IIIa expression and platelet-leukocyte aggregate formation. Am. J. Cardiol. 90:312–315 (2002).
J. E. Mobley, S. J. Bresee, D. C. Wortham, et al. Frequency of nonresponse antiplatelet activity of clopidogrel during pretreatment for cardiac catheterization. Am. J. Cardiol. 93:456–458 (2004).
P. A. Gurbel, and K. P. Bliden. A new method of representing drug-induced platelet inhibition: better description of time course, response variability, non-response, and heightened activity. Platelets 14:481–483 (2003).
P. Jaremo, T. L. Lindahl, S. G. Fransson, et al. Individual variations of platelet inhibition after loading doses of clopidogrel. J. Intern. Med. 252:233–238 (2002).
P. A. Gurbel, and K. P. Bliden. Durability of platelet inhibition by clopidogrel. Am. J. Cardiol. 91:1123–1125 (2003).
Y. Uno, Y. Sakamoto, K. Yoshida, et al. Characterization of six base pair deletion in the putative HNF1-binding site of human PXR promoter. J. Hum. Genet. 48:594–597 (2003).
W. C. Lau. St. John's wort enhances the platelet inhibitory effect of clopidogrel in clopidogrel “Resistant” healthy volunteers. J. Am. Coll. Cardiol. 45:382A (2005) (Abstract).
I. Muller, M. Seyfarth, S. Rudiger, et al. Effect of a high loading dose of clopidogrel on platelet function in patients undergoing coronary stent placement. Heart 85:92–93 (2001).
A. Kastrati, J. Mehilli, H. Schuhlen, et al. A clinical trial of abciximab in elective percutaneous coronary intervention after pretreatment with clopidogrel. N. Engl. J. Med. 350:232–238 (2004).
W. C. Lau, J. M. Rae, P. F. Hollenberg et al. Clopidogrel is an inducer and a potent reversible inhibitor of cytochrome P450 3A4 in vitro. J. Am. Coll. Cardiol. 43:493A (2004).
J. Saw, S. R. Steinhubl, P. B. Berger, et al. Lack of adverse clopidogrel–atorvastatin clinical interaction from secondary analysis of a randomized, placebo-controlled clopidogrel trial. Circulation 108:921–924 (2003).
V. L. Serebruany, M. G. Midei, A. I. Malinin, et al. Absence of interaction between atorvastatin or other statins and clopidogrel: results from the interaction study. Arch. Intern. Med. 164:2051–2057 (2004).
H. Wienbergen, A. K. Gitt, R. Schiele, et al. Comparison of clinical benefits of clopidogrel therapy in patients with acute coronary syndromes taking atorvastatin versus other statin therapies. Am. J. Cardiol. 92:285–288 (2003).
J. Brophy, V. Costa, and M. Babapulle. A pharmaco-epidemiological study of the interaction between atorvastatin and clopidogrel following percutaneous coronary interventions. J. Am. Coll. Cardiol. 43:50A (2004) (Abstract).
M. Blumenthal (ed.), The complete German Commission E monographs-therapeutic guide to herbal medicines. American Botanical Council, 1998.
R. E. Watkins, J. M. Maglich, L. B. Moore, et al. 2.1 A crystal structure of human PXR in complex with the St. John's wort compound hyperforin. Biochemistry 42:1430–1438 (2003).
L. B. Moore, B. Goodwin, S. A. Jones, et al. St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc. Natl. Acad. Sci. U. S. A. 97:7500–7502 (2000).
A. Biber, H. Fischer, A. Romer, et al. Oral bioavailability of hyperforin from hypericum extracts in rats and human volunteers. Pharmacopsychiatry 31(Suppl 1):36–43 (1998).
C. A. Roby, G. D. Anderson, E. Kantor, et al. St John's Wort: effect on CYP3A4 activity. Clin. Pharmacol. Ther. 67:451–457 (2000).
J. McEwen, G. Strauch, P. Perles, et al. Clopidogrel bioavailability: absence of influence of food or antacids. Semin. Thromb. Hemost. 25(Suppl 2):47–50 (1999).
N. von Beckerath, D. Taubert, G. Pogatsa-Murray, et al. Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) trial. Circulation 112:2946–2950 (2005).
D. Taubert, A. Kastrati, S. Harlfinger, et al. Pharmacokinetics of clopidogrel after administration of a high loading dose. Thromb. Haemost. 92:311–316 (2004).
E. Wang, C. N. Casciano, R. P. Clement, et al. HMG-CoA reductase inhibitors (statins) characterized as direct inhibitors of P-glycoprotein. Pharm. Res. 18:800–806 (2001).
K. Bogman, A. K. Peyer, M. Torok, et al. HMG-CoA reductase inhibitors and P-glycoprotein modulation. Br. J. Pharmacol. 132:1183–1192 (2001).
T. Sakaeda, K. Takara, M. Kakumoto, et al. Simvastatin and lovastatin, but not pravastatin, interact with MDR1. J. Pharm. Pharmacol. 54:419–423 (2002).
P. Fontana, A. Dupont, S. Gandrille, et al. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation 108:989–995 (2003).
D. J. Angiolillo, A. Fernandez-Ortiz, E. Bernardo, et al. Lack of association between the P2Y(12) receptor gene polymorphism and platelet response to clopidogrel in patients with coronary artery disease. Thromb. Res. 116:491–497 (2005).
A. Sugidachi, F. Asai, T. Ogawa, et al. The in vivo pharmacological profile of CS-747, a novel antiplatelet agent with platelet ADP receptor antagonist properties. Br. J. Pharmacol. 129:1439–1446 (2000).
Y. Niitsu, J. A. Jakubowski, A. Sugidachi, et al. Pharmacology of CS-747 (prasugrel, LY640315), a novel, potent antiplatelet agent with in vivo P2Y12 receptor antagonist activity. Semin. Thromb. Hemost. 31:184–194 (2005).
S. D. Wiviott, E. M. Antman, K. J. Winters, et al. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the joint utilization of medications to block platelets optimally (JUMBO)-TIMI 26 trial. Circulation 111:3366–3373 (2005).
F. Storey. The P2Y12 receptor as a therapeutic target in cardiovascular disease. Platelets 12:197–209 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lau, W.C., Gurbel, P.A. Antiplatelet Drug Resistance and Drug-Drug Interactions: Role of Cytochrome P450 3A4. Pharm Res 23, 2691–2708 (2006). https://doi.org/10.1007/s11095-006-9084-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9084-4