Abstract
Abstract
This study aimed to investigate the allele frequencies of CYP2B6 gene in 193 Han Chinese and compared with 91 Uygur Chinese. Five single nucleotide polymorphisms of CYP2B6, 64C>T, 516G>T, 777C>A, 785A>G and 1459C>T, were tested using the polymerase chain reaction–restriction fragment length polymorphism method. The allele frequencies for CYP2B6*2, CYP2B6*3, CYP2B6*4, CYP2B6*5, CYP2B6*6, CYP2B6*7 and CYP2B6*9 in Han and Uygur Chinese were 0.034 and 0.027, 0 and 0.011, 0.091 and 0.033, 0.003 and 0.049, 0.184 and 0.214, 0 and 0.022, and 0.018 and 0.044, respectively, with CYP2B6*4, CYP2B6*5, and CYP2B6*7 being significantly different between these two races (P<0.05). CYP2B6*6 was the most prevalent allele among all detected variants in Han and Uygur Chinese. The most frequent genotypes were CYP2B6*1/CYP2B6*1 (50.8%), CYP2B6*1/CYP2B6*6 (24.4%), and CYP2B6*1/CYP2B6*4 (7.3%) in Han subjects, whereas the most frequent genotypes in Uygur subjects were CYP2B6*1/CYP2B6*1 (36.3%), CYP2B6*1/CYP2B6*6 (25.3%), CYP2B6*1/CYP2B6*5 (5.5%) and CYP2B6*6/CYP2B6*6 (5.5%). The frequencies of 64C>T mutation in Han and Uygur Chinese were significantly lower than that in American Caucasian (P<0.05). These results indicate that there were marked ethnic differences in the mutant frequencies of CYP2B6 between Chinese and other ethnic groups. Further studies are warranted to explore the clinical impact of such ethnic differences.
Similar content being viewed by others
Introduction
The cytochrome P450s (CYPs) are the most important Phase I metabolizing enzymes which metabolize a variety of xenobiotics including carcinogens and therapeutic drugs and some important endogenous substances (e.g., steroids, hormones, and fatty acids) (1). CYP2B6 was found at 3–5% of total hepatic CYP content in 20–100% of humans (2), and consistently, it was responsible for the metabolism of about 3% of therapeutic drugs, a few endogenous substances, some environmental compounds and procarcinogens (1). The drugs metabolized primarily or partially by this enzyme include cyclophosphamide (3), promazine (4), methadone (5,6), S-mephenytoin (7), nevirapine (8), efavirenz (9), propofol (10), and bupropion (11). Furthermore, human CYP2B6 also hydroxylates testosterone and androstenedione (12,13). CYP2B6 is mainly expressed in human liver and some extra-hepatic tissues as well, including the kidney, intestine, brain, skin, and lung at a lower level (14). Notably, there are a very wide (20- to 288-fold) individual variability in CYP2B6 protein expression and enzyme activity levels (15–17). Such wide variability may be due to environmental factors and polymorphisms of the CYP2B6 gene. In contrast, CYP2B6 mRNA levels showed less variability (13-fold) (15). However, it is difficult to evaluate the in vivo activity of CYP2B6 in humans because of the lack of specific substrates. It appears that bupropion, an antidepressant and antismoking agent, is one of the most specific substrates of CYP2B6 and, therefore, is commonly used as an in vitro probe drug for this enzyme (11). Recently, several highly specific inhibitors for CYP2B6 have been found, including the thiotepa (18,19), 17-α-ethynylestradiol (20), and clopidogrel (21). All these probe compounds are useful tools in the functional studies of CYP2B6 in vitro and in vivo.
The human CYP2B6 gene has been mapped to chromosome 19 between 19q12 and 19q13.2 and is composed of nine exons (22). Up to date, a number of single nucleotide polymorphisms (SNPs) have been reported in CYP2B6 gene (http://www.imm.ki.se/CYPalleles). Lang et al. (23) first identified nine SNPs of CYP2B6 in Caucasian (German), with five of which leading to amino acid substitutions. In this study, six other CYP2B6 alleles have been found, namely, CYP2B6*2 (64C>T), CYP2B6*3 (777C>A), CYP2B6*4 (785A>G), CYP2B6*5 (1459C>T), CYP2B6*6 (516G>T and 785A>G), CYP2B6*7 (516G>T, 785A>G and 1459C>T) (23). Thereafter, Lamba et al. (24) reported two new SNPs, CYP2B6*8 (415A>G resulting in an Lys139Glu) and CYP2B6*9 (516G>T). In addition, many SNPs in the CYP2B6 promoter region, such as −1848C>A, −801G>T, −750T>C, and −82T>C, have been identified (24,25). Recently, eight new missense mutations (76A>T, 83A>G, 85C>A, 86G>C, 15618C>T, 18038G>A, 21034C>, 21498C>A, three new silent mutations and two new intronic SNPs defining six novel alleles (CYP2B6*17A and CYP2B6*17B, CYP2B6*18, CYP2B6*19, CYP2B6*20, CYP2B6*21) have been identified (26). More recently, haplotype analysis in 51 African HIV patients revealed that 983T>C was linked with 785A>G defining a novel allele, CYP2B6*16 (27). This allele was present in totally five of the patients (9.8%). The CYP2B6*16 allele was not found in Swedes, was present at 4% frequency among Turks (27). Many of these SNPs in CYP2B6 have an impact on the enzyme activity. For example, a 1459C>T (Arg487Cys) SNP in exon 9 caused a 8-fold lower enzyme activity compared to the wild-type protein (23). On the other hand, the subjects with CYP2B6*4 (Lys262Arg), CYP2B6*5 (Arg487Cys), CYP2B6*6 (Gln172His and Lys262Arg), or CYP2B6*7 (Gln172His, Lys262Arg, and Arg487Cys) genotype had a higher enzyme activity to 7-ethoxy-4-trifluoromethylcoumarin, compared to CYP2B6*1 (wild type) (28). CYP2B6*6 (Gln172His and Lys262Arg) was associated with increased 4-hydroxylation of cyclophosphamide in vitro (29). Moreover, the −82T>C mutation in the TATA box of the promoter region of CYP2B6 gene resulted in approximately two–fold higher enzyme activity toward bupropion compared to the wild-type protein (25). However, CYP2B6*2 (Arg22Cys) and CYP2B6*16 with the 785A>G SNP did not alter the CYP2B6 activity (27,28).
The frequencies of variant alleles of CYP2B6 gene have been studied in Caucasian, African, Japanese and Korean, but no data are available for the Chinese population except for our recent report (30). As to CYP2B6, it is especially important to determine all of the genetic variants responsible for altered CYP2B6 expression in different ethnic groups, because there are no validated and specific drugs that can currently be used as model substrates to probe CYP2B6 activity in vivo and to guide dosing of CYP2B6 substrate drugs with narrow therapeutic indices. This prompted us to investigate the allele frequencies of common CYP2B6 alleles, including 64C>T, 516G>T, 777C>A, 785A>G, and 1459C>T, in Han Chinese and compared with Uygur Chinese. The Han Chinese is predominant among all 56 different racial groups in China, numbering 1.159 billion and making up 91.59% of the country's population. Uygur Chinese is a minority in China with a total population of 1.6 million. Since Uygur Chinese is ethnically belongs to Caucasians, we chose this ethnic group as a control group. The resultant genetic data in Chinese were also compared with those in Caucasian, African, Japanese and Korean reported in literatures.
Materials and Methods
Study Population
Venous blood samples were obtained from unrelated healthy Han (n = 193; 134 males and 59 females; age ranging from 20 to 33 years; mean ± SD 24.1 ± 1.6 years) and Uygur Chinese volunteers (n = 91; 21 males and 70 females; age ranging from 17 to 21 years; mean 18.4 ± 4.2 years). The volunteers were from Sun Yat-sen University, Guangdong Province, and Professional Technology College of Xinjiang Uighur Autonomous Region, China. The ethnicity of all volunteers was confirmed by their social and culture characteristics and official identity information. Ethical approval of this study was obtained from the Ethical committee of Sun Yat-sen University, Guangzhou, China and written informed consent was obtained from all participants.
Molecular Studies
Specific intronic polymerase chain reaction (PCR) primer pairs used in the human CYP2B6 genetic mutation screening were designed using CYP2B6 genomic sequences retrieved from GenBank (accession no. AC023172.1; GI: 6957691) [GenBank]. Exon–intron boundaries of the human CYP2B6 gene were defined by comparing genomic sequences with the mRNA sequence (accession no. M29874.1) [GenBank] (31).
Total genomic DNA was extract from peripheral leukocytes by the phenol–chloroform extraction method (32). Their purity and concentration were examined. The polymerase chain reaction–restriction fragment length polymorphism (RFLP) assay for detecting CYP2B6 mutations was as described by Lang et al. (23). The primers used to detect the SNPs, 64C>T, 516G>T, 777C>A, 785A>G, and 1459C>T, on exons 1, 4, 5 and 9, respectively, are listed in Table I.
To detect 64C>T SNP on exon 1, the PCR reactions were performed in a total volume of 50 μl with 100 ng genomic DNA, 2.5 mM dNTPs, 10 μM of primer CYP2B6-1F and primer CYP2B6-1R (see Table I), 5 μl 10× Ex Taq buffer and 1.25 U Ex Taq DNA polymerase (Takara, Japan). After 5 min of denaturation at 95°C, the PCR mixtures were subjected to the following conditions: 30 s at 95°C, 30 s at 50°C and 1 min at 72°C for 35 cycles with a delayed last step of 10 min at 72°C in a PCR System 2700 (Applied Biosystems, Foster City, CA, USA). Aliquots of each sample were digested with HaeII (New England Biolabs, Beverly, MA, USA), and the resulting fragments were analyzed on a 2.0% agarose gel (Shanghai Yito Bio-Instrument Company Limited, Shanghai, P.R.China) stained with ethidium bromide. HaeII digestion of wild-type DNA yielded fragments of 333 and 390 bp, whereas the 64C>T mutation prevented enzyme digestion and resulted in an uncleaved fragment of 723 bp (Fig. 1a).
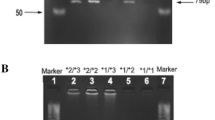
Representative electrophoresis gel photos showing the five single nucleotide polymorphisms, 64C>T (a), 516G>T (b), 777C>A (c), 785A>G (d), and 1459C>T (e), in CYP2B6 gene in Chinese, using PCR_RFLP methods. Typical PCR reactions were conducted in a reaction volume of 50 μl with 20 ng genomic DNA, 10× PCR buffer, 200 μM dNTPs, 10 pmol of each primer, and 1 unit of polymerase. The resultant DNA fragments were separated by electrophoresis in 2.0–2.5% agarose gel. Each mutation gave rise to different DNA fragments from those in the wild type. w, wild type; m, mutation
For amplification of exon 4 to detect 516G>T mutation, the primer pair CYP2B6-4F/-4R was used with the same cycling and analysis conditions as for exon 1, except that 56°C was used as annealing temperature and the extension step was reduced to 40 s. The purified PCR products were digested with restriction enzyme BsrI (New England Biolabs, Beverly, MA, USA). The wild-type DNA resulted in three fragments of 241, 268 and 17 bp, whereas mutant PCR products resulted in two fragments of 509 and 17 bp (Fig. 1b).
To detect 777C>A SNP on exon 5, the DNA was amplified with primers CYP2B6-5F/-5R using the same cycling conditions as for exon 11 except that the annealing temperature was 60°C. The mutation was detected by digestion with restriction enzyme HaeII, leaving mutated DNA at position 777 undigested. The wild-type restriction fragments were 140, 196 and 304 bp, whereas mutated samples resulted in 500- and 140-bp fragments (Fig. 1c).
The same PCR fragment used in the detection of the 777C>A mutation was analysed for the 785A>G mutation in exon 5 which disrupted a recognition sequence for StyI (New England Biolabs, Beverly, MA, USA). Digestion of the wild-type DNA resulted in fragments of 56, 116, 171 and 297 bp. Presence of the 785A>G mutation gave three fragments of 56, 116 and 468 bp. The fragments were separated using a 2.5% agarose gel (Shanghai Yito Bio-Instrument Company Limited, Shanghai, People's Republic of China) to resolve fragments of 171 and 116 bp (Fig. 1d).
In addition, exon 9 was amplified with primers CYP2B6-9F/-9R using the same conditions as for exon 1 except that a prolonged extension step of 1 min 30 s and an annealing step at 58.5°C were used. The 1459C>T mutation resulted in a restriction enzyme site for BglII (New England Biolabs, Beverly, MA, USA), giving rise to two fragments of 216 and 1185 bp. The wild-type DNA yielded a PCR fragment of 1,401 bp which was not cleaved (Fig. 1e).
If the mutations were detected by PCR–RFLP assay in any subjects, the amplified DNA fragments were further sequenced to confirm the mutations using an ABI 3700 DNA sequencer (Applied Biosystems, Foster City, CA, USA) with the BigDye RR Terminator Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA).
For CYP2B6 variants, genotyping of 64C>T (CYP2B6*2A, CYP2B6*2B, CYP2B6*10), 516G>T (CYP2B6*6A, CYP2B6*6B, CYP2B6*6C, CYP2B6*7A, CYP2B6*7B, CYP2B6*9, CYP2B6*13A, CYP2B6*13B, CYP2B6*19, and CYP2B6*20), 777C>A (CYP2B6*3), 785A>G (CYP2B6*4A, CYP2B6*4B, CYP2B6*4C, CYP2B6*4D, CYP2B6*6A, CYP2B6*6B, CYP2B6*6C, CYP2B6*7A, CYP2B6*7B, CYP2B6*13B, CYP2B6*16, CYP2B6*19, and CYP2B6*20), and 1459C>T (CYP2B6*5A, CYP2B6*5B, CYP2B6*5C, and CYP2B6*7) was carried out.
Nomenclature and Statistical Analysis
Allele designations were made in accordance with the Human CYPallele Nomenclature Committee (http://www.imm.ki.se/CYPalleles/criteria.htm). Numbering was based on the cDNA with the full-length cDNA sequence published by Yamano et al. (31) defined as the wild type (CYP2B6*1). Genomic sequence numbers are available at the CYP allele nomenclature website.
Analysis of data was performed by using the computer software SPSS for Windows (Version 12.0). CYP2B6*1 alleles were counted when the point mutations tested in the present study did not exist. The frequency of each allele in our study population is given together with the 95% confidence interval (CI). The lower and upper limits of the 95% CI for allele frequencies of CYP2B6 were determined as described by Newcombe (33) with a correction for continuity. The effects of gender and ethnicity on the frequencies of CYP2B6 genotypes were tested using χ 2 test with Yates' continuity correction or Fisher's exact test. Values of P<0.05 were considered significant.
Hardy-Weinberg principle means that the genotype frequencies at a single gene locus will become fixed at a particular equilibrium value under certain conditions, after one generation of random mating; and it also specifies that those equilibrium frequencies can be represented as a simple function of the allele frequencies at that locus. Whenever appropriate, the observed number of each single point mutation (instead of alleles, i.e. haplotypes) was compared with that expected for a population in the Hardy–Weinberg equilibrium by using a goodness-of-fit χ 2 test. Values of P<0.05 were considered significant.
Results and Discussion
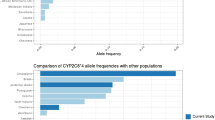
The data for CYP2B6 allele frequencies in both Han and Uygur Chinese are shown in Table II. There were no significant differences in the frequencies of CYP2B6*1, CYP2B6*2, CYP2B6*3, CYP2B6*6 and CYP2B6*9 between Han Chinese (67.1, 3.4, 0, 18.4 and 1.8%, respectively) and Uygur Chinese (59.9, 2.7, 1.1, 21.4 and 4.4%, respectively) (P=0.109, 0.802, 0.102, 0.426 and 0.092, respectively). However, for CYP2B6*4, Han Chinese had a significantly higher frequency (9.1%) than Uygur Chinese (3.3%) (P=0.014), whereas those of alleles CYP2B6*5 and CYP2B6*7 were lower in Han Chinese (0.3 and 0%, respectively) than in Uygur Chinese (4.9 and 2.2%, respectively) (P=0.010 and P<0.001, respectively). CYP2B6*6 was most prevalent among all detected variant alleles in Han and Uygur Chinese.
Table III shows the distribution of various CYP2B6 genotypes in Han and Uygur Chinese. The most frequent genotypes were CYP2B6*1/*1 (50.8%), followed by CYP2B6*1/*6 (24.4%), and CYP2B6*1/*4 (7.3%) in Han Chinese, whereas the most frequent genotype in Uygur subjects was CYP2B6*1/*1 (36.3%), followed by CYP2B6*1/*6 (25.3%), CYP2B6*1/*5 (5.5%) and CYP2B6*6/*6 (5.5%). These results were in good accordance with the Hardy–Weinberg equilibrium. Different from Han Chinese, Uygur Chinese belongs to Caucasians. The frequencies of main mutant CYP2B6 alleles in Uygur Chinese were between those in Han Chinese and those in Caucasians, and more similar to those in Caucasians. The little discrepancy between Uygur and Caucasians is unknown. From an anthropological point of view, geographical factors and mingling of Uygur people with Han Chinese and other ethnic groups may contribute to such discrepancy.
As shown in Table IV, the frequency of the SNP 1459C>T in Uygur Chinese was significantly higher than that in Han Chinese (P<0.05) and Japanese (P<0.05) (34). The frequencies of the SNP 64C>T in Han and Uygur Chinese were significantly lower than that in American Caucasian (P<0.05) (24). For the SNP 516G>T, the frequency in Uygur Chinese was higher than that in Japanese (P<0.05) (34); and the frequency in Han Chinese was lower than that in German Caucasian (P<0.05) (23). In addition, the frequencies of the SNP 785A>G in Han and Uygur Chinese were significantly higher than that in American Caucasian (P<0.05) (24). However, there was no significant difference of the frequency of SNP 777C>A among the five ethnic groups. All of the above polymorphisms were in Hardy-Weinberg equilibrium in all ethnic groups examined.
Ethnicity is an important variable contributing to interindividual variability in drug metabolism, response and toxicity (35–37). In recent years, a number of SNPs in CYP genes have been found to account for interethnic variations in response to a large number of therapeutic agents. The frequency of functional SNPs varies widely between ethnic groups and the Asian population is no exception to this. However, most of the current literature is concerned with polymorphisms of CYP genes including CYP2B6 in Caucasians and African-Americans, whereas the data in Asian populations, in particular Chinese, are scanty. Thus, we studied the allele frequencies of common CYP2B6 in Han and Uygur Chinese, for the first time, and compared with those in other ethic groups reported in the literature. In the present study, 1.1% (not significantly compared to Han Chinese) of variant CYP2B6*3 and 2.2% CYP2B6*7 (P<0.0001, compared to Han Chinese) were identified in 91 Uygur Chinese whereas no subjects with variant CYP2B6*3 and CYP2B6*7 alleles were detected among 193 Han Chinese. These results suggested the ethnic differences did exist between these two groups. The frequency of Han Chinese was in agreement with that of other Asians including Japanese and Korean (34,38), and the frequency of Uygur Chinese was consistent with that of Caucasians (23,24).
The frequencies of variants CYP2B6*5 (P=0.010), CYP2B6*6 (not significantly) were similar to those of CYP2B6*3 and CYP2B6*7 between Han and Uygur Chinese. In contrast, the frequency of variant CYP2B6*4 in Han Chinese was significantly higher (P=0.014) than that in Uygur Chinese. It was not a surprise that the frequency of CYP2B6*4 in Uygur Chinese was similar to those reported for Caucasian populations (23); whereas, the observed frequency in Han Chinese was consistent with data reported for other Asians including Japanese and Korean (34,38). However, in this study, one 516G>T allele was found in both Han Chinese and Uygur Chinese populations. This was different from data of Japanese population (34) and Caucasian (German) population (23). This mutant allele was recently named of CYP2B6*9 by Lamba et al. (24). As for CYP2B6*2, there were no significant differences among all these ethnic groups.
The overall frequencies of variant CYP2B6 alleles in Uygur Chinese were higher than that in Han Chinese. The SNP of 785A>G seemed to be the most prevalent CYP2B6 allele in both Han (allele frequency 28%) and Uygur (allele frequency 27%) Chinese and 777C>A appeared to be a rare allele (allele frequency of 0 and 1%, respectively) in these two ethnic groups. There were no significantly statistical differences of all the SNPs except 1459C>T between Han and Uygur Chinese population. The frequencies of 64C>T and 785A>G in Uygur Chinese were similar to those in Han Chinese, Japanese (34) and German Caucasian (23); however, their frequencies were significantly different from that of American Caucasians (24). For 1459C>T, the frequency in Uygur Chinese was markedly higher than that in Han Chinese and Japanese population (34), but significantly lower than that of Caucasians (23,24). Furthermore, the overall frequencies of mutant alleles in Chinese (52.3%) are lower than those in German Caucasians (81.5%), but higher than American Caucasians (48.0%). The reason for the difference in the overall frequency of mutant alleles between German and American is unknown, but may be associated with the fact the frequency of 785A>G was 33% in Germans whereas it is only 4% in Americans. Because of unknown reasons, German have much higher 785A>G incidence than American people.
The marked ethnic differences in the frequencies of CYP2B6 mutations may have important clinical implications. The related variant CYP2B6*6 (Q172H and K262R) was found to be associated with increased 4-hydroxylation of cyclophosphamide in vitro (44) and this may provide partial explanation for the large (15- to 50-fold) interpatient differences observed in the elimination of cyclophosphamide (44–46). Moreover, some CYP2B6 mutations have a major impact on the pharmacokinetics and pharmacodynamics of in HIV patients (39, 41). Lower plasma efavirenz clearance and increased central nervous system side effects are associated with the SNP 516G>T of CYP2B6 that is more frequent among African American individuals than among European American individuals (42, 43), but this SNP appears not to alter long-term response to efavirenz therapy in HIV patients (40). Wang et al. (27) recently reported that the steady-state level of efavirenz was significantly higher in African carriers of CYP2B6*16, compared to the other patients. Higher efavirenz plasma concentrations were also seen in carriers of 516G>T SNP in this study population (27). To better understand the clinical implications of in vivo phenotypic differences in metabolism of the CYP2B6 enzyme, determination of the relationship between each genotype of the CYP2B6 gene and the enzymatic activity of CYP2B6 and their interplay with smoking, alcohol and other factors in Han Chinese will be of interest. However, although some clinically important drugs are substrates of CYP2B6, the overall importance for this enzyme in the metabolism of therapeutic compounds is small compared to CYP3A4, 2D6 and 2C9.
Although there are no significantly statistical differences, the frequencies of mutations in female are higher than those in male in Han Chinese. This is consistent with that reported for the Caucasian population (24). It is expected that increasing sample size may find a significant effect of gender on CYP2B6 allele frequency. Such gender differences may result in significant differences in CYP2B6 expression in liver between sexes. Indeed, marked gender differences in CYP2B6 expression and activity have been observed in several studies in the Caucasian, African, or Hispanic Americans (24). Females had higher amounts of CYP2B6 mRNA, protein, and activity than did male subjects (24). Twenty percent of the males but only 7.1% of females were poor CYP2B6 metabolizers. This difference appears likely to be translated to humans in vivo because a difference between sexes in metabolism of ifosfamide, a CYP2B6 substrate, has been reported (47). The relative level of constitutive and rostane receptor gene mRNA, a critical regulator of CYP2B6, is higher in females than in males, and could directly result in gender differences in the rate of CYP2B6 transcription (48).
In this study, only five common non-synonymous mutant alleles in exons of CYP2B6, 64C>T, 516G>T, 777C>A, 785A>G and 1459C>T, were genotyped in Han Chinese. We inferred that the samples in which these mutant alleles were not detected had the wild-type CYP2B6*1. However, there remains a possibility of the presence of other mutant allele, such as some synonymous SNPs in other region including promoter region, some introns and other exons, and some splice variants (49). It can be predicted some mutations exist in the promoter region of CYP2B6 gene in Han Chinese and thus further studies are needed to screen these reported mutation found in Caucasians due to their important functional impact on enzyme activity. Another limitation of the present study is the lack of function and structure–activity analysis of CYP2B6 mutations in Han Chinese. A recent site-directed mutagenesis study indicated that substitution of Leu-363 with valine in CYP2B6 reduced the ω-1 hydroxylation of 7-butoxycoumarin significantly and increased O-dealkylation (50).
Overall, our finding in Han Chinese and Uygur Chinese provides further evidence for racial heterogeneity in CYP2B6 mutations in Chinese. Such intra-ethnic difference is also marked between Chinese and Caucasian and African. Detailed pharmacogenetic studies of CYP2B6 gene may offer a preliminary basis for rational use of CYP2B6 substrate drugs in Chinese.
Abbreviations
- CI:
-
confidence interval
- PCR:
-
polymerase chain reaction
- RFLP:
-
restriction fragment length polymorphism
- SNP:
-
single nucleotide polymorphism
References
S. Rendic. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab. Rev. 34:83–448 (2002).
S. Ekins, M. VandenBranden, B. J. Ring, and S. A. Wrighton. Examination of purported probes of human CYP2B6. Pharmacogenetics 7:165–179 (1997).
T. K. Chang, G. F. Weber, C. L. Crespi, and D. J. Waxman. Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res. 53:5629–5637 (1993).
J. Wojcikowski, L. Pichard-Garcia, P. Maurel, and W. A. Daniel. Contribution of human cytochrome p-450 isoforms to the metabolism of the simplest phenothiazine neuroleptic promazine. Br. J. Pharmacol. 138:1465–1474 (2003).
J. G. Gerber, R. J. Rhodes, and J. Gal. Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19. Chirality 16:36–44 (2004).
Y. Oda and E. D. Kharasch. Metabolism of levo-alpha-Acetylmethadol (LAAM) by human liver cytochrome P450: involvement of CYP3A4 characterized by atypical kinetics with two binding sites. J. Pharmacol. Exp. Ther. 297:410–422 (2001).
H. Heyn, R. B. White, and J. C. Stevens. Catalytic role of cytochrome P4502B6 in the N-demethylation of S-mephenytoin. Drug Metab. Dispos. 24:948–954 (1996).
P. Riska, M. Lamson, T. MacGregor, J. Sabo, S. Hattox, J. Pav, and J. Keirns. Disposition and biotransformation of the antiretroviral drug nevirapine in humans. Drug Metab. Dispos. 27:895–901 (1999).
B. A. Ward, J. C. Gorski, D. R. Jones, S. D. Hall, D. A. Flockhart, and Z. Desta. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J. Pharmacol. Exp. Ther. 306:287–300 (2003).
M. H. Court, S. X. Duan, L. M. Hesse, K. Venkatakrishnan, and D. J. Greenblatt. Cytochrome P-450 2B6 is responsible for interindividual variability of propofol hydroxylation by human liver microsomes. Anesthesiology 94:110–119 (2001).
S. R. Faucette, R. L. Hawke, E. L. Lecluyse, S. S. Shord, B. Yan, R. M. Laethem, and C. M. Lindley. Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab. Dispos. 28:1222–1230 (2000).
T. L. Domanski, K. M. Schultz, F. Roussel, J. C. Stevens, and J. R. Halpert. Structure–function analysis of human cytochrome P-450 2B6 using a novel substrate, site-directed mutagenesis, and molecular modeling. J. Pharmacol. Exp. Ther. 290:1141–1147 (1999).
T. J. Yang, K. W. Krausz, M. Shou, S. K. Yang, J. T. Buters, F. J. Gonzalez, and H. V. Gelboin. Inhibitory monoclonal antibody to human cytochrome P450 2B6. Biochem. Pharmacol. 55:1633–1640 (1998).
L. Gervot, B. Rochat, J. C. Gautier, F. Bohnenstengel, H. Kroemer, V. de Berardinis, H. Martin, P. Beaune, and I. de Waziers. Human CYP2B6: expression, inducibility and catalytic activities. Pharmacogenetics 9:295–306 (1999).
L. M. Hesse, P. He, S. Krishnaswamy, Q. Hao, K. Hogan, L. L. von Moltke, D. J. Greenblatt, and M. H. Court. Pharmacogenetic determinants of interindividual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes. Pharmacogenetics 14:225–238 (2004).
H. Kim, R. S. Wang, E. Elovaara, H. Raunio, O. Pelkonen, T. Aoyama, H. Vainio, and T. Nakajima. Cytochrome P450 isozymes responsible for the metabolism of toluene and styrene in human liver microsomes. Xenobiotica 27:657–665 (1997).
S. Ekins, M. Vandenbranden, B. J. Ring, J. S. Gillespie, T. J. Yang, H. V. Gelboin, and S. A. Wrighton. Further characterization of the expression in liver and catalytic activity of CYP2B6. J. Pharmacol. Exp. Ther. 286:1253–1259 (1998).
J. M. Rae, N. V. Soukhova, D. A. Flockhart, and Z. Desta. Triethylenethiophosphoramide is a specific inhibitor of cytochrome P450 2B6: implications for cyclophosphamide metabolism. Drug Metab. Dispos. 30:525–530 (2002).
T. Richter, M. Schwab, M. Eichelbaum, and U. M. Zanger. Inhibition of human CYP2B6 by N,N′,Ná-triethylenethiophosphoramide is irreversible and mechanism-based. Biochem. Pharmacol. 69:517–524 (2005).
U. M. Kent, D. E. Mills, R. V. Rajnarayanan, W. L. Alworth, and P. F. Hollenberg. Effect of 17-alpha-ethynylestradiol on activities of cytochrome P450 2B (P450 2B) enzymes: characterization of inactivation of P450s 2B1 and 2B6 and identification of metabolites. J. Pharmacol. Exp. Ther. 300:549–558 (2002).
T. Richter, T. E. Murdter, G. Heinkele, J. Pleiss, S. Tatzel, M. Schwab, M. Eichelbaum, and U. M. Zanger. Potent mechanism-based inhibition of human CYP2B6 by clopidogrel and ticlopidine. J. Pharmacol. Exp. Ther. 308:189–197 (2004).
I. Santisteban, S. Povey, E. A. Shephard, and I. R. Phillips. The major phenobarbital-inducible cytochrome P-450 gene subfamily (P450IIB) mapped to the long arm of human chromosome 19. Ann. Hum. Genet. 52(Pt 2):129–135 (1988).
T. Lang, K. Klein, J. Fischer, A. K. Nussler, P. Neuhaus, U. Hofmann, M. Eichelbaum, M. Schwab, and U. M. Zanger. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics 11:399–415 (2001).
V. Lamba, J. Lamba, K. Yasuda, S. Strom, J. Davila, M. L. Hancock, J. D. Fackenthal, P. K. Rogan, B. Ring, S. A. Wrighton, and E. G. Schuetz. Hepatic CYP2B6 expression: gender and ethnic differences and relationship to CYP2B6 genotype and CAR (constitutive androstane receptor) expression. J. Pharmacol. Exp. Ther. 307:906–922 (2003).
J. Zukunft, T. Lang, T. Richter, K. I. Hirsch-Ernst, A. K. Nussler, K. Klein, M. Schwab, M. Eichelbaum, and U. M. Zanger. A natural CYP2B6 TATA box polymorphism (-82T–>C) leading to enhanced transcription and relocation of the transcriptional start site. Mol. Pharmacol. 67:1772–1782 (2005).
K. Klein, T. Lang, T. Saussele, E. Barbosa-Sicard, W. H. Schunck, M. Eichelbaum, M. Schwab, and U. M. Zanger. Genetic variability of CYP2B6 in populations of African and Asian origin: allele frequencies, novel functional variants, and possible implications for anti-HIV therapy with efavirenz. Pharmacogenet. Genomics 15:861–873 (2005).
J. Wang, A. Sonnerborg, A. Rane, F. Josephson, S. Lundgren, L. Stahle, and M. Ingelman-Sundberg. Identification of a novel specific CYP2B6 allele in Africans causing impaired metabolism of the HIV drug efavirenz. Pharmacogenet. Genomics 16:191–198 (2006).
H. Jinno, T. Tanaka-Kagawa, A. Ohno, Y. Makino, E. Matsushima, N. Hanioka, and M. Ando. Functional characterization of cytochrome P450 2B6 allelic variants. Drug Metab. Dispos. 31:398–403 (2003).
N. Ariyoshi, M. Miyazaki, K. Toide, Y. Sawamura, and T. Kamataki. A single nucleotide polymorphism of CYP2b6 found in Japanese enhances catalytic activity by autoactivation. Biochem. Biophys. Res. Commun. 281:1256–1260 (2001).
S. Guan, M. Huang, E. Chan, X. Chen, W. Duan, and S.F. Zhou. Genetic polymorphisms of cytochrome P450 2B6 gene in Han Chinese. Eur J Pharm Sci. (in press)
S. Yamano, P. T. Nhamburo, T. Aoyama, U. A. Meyer, T. Inaba, W. Kalow, H. V. Gelboin, O. W. McBride, and F. J. Gonzalez. cDNA cloning and sequence and cDNA-directed expression of human P450 IIB1: identification of a normal and two variant cDNAs derived from the CYP2B locus on chromosome 19 and differential expression of the IIB mRNAs in human liver. Biochemistry 28:7340–7348 (1989).
P. Chomczynski and N. Sacchi. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 162:156–159 (1987).
R. G. Newcombe. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat. Med. 17:857–872 (1998).
M. Hiratsuka, Y. Takekuma, N. Endo, K. Narahara, S. I. Hamdy, Y. Kishikawa, M. Matsuura, Y. Agatsuma, T. Inoue, and M. Mizugaki. Allele and genotype frequencies of CYP2B6 and CYP3A5 in the Japanese population. Eur. J. Clin. Pharmacol. 58:417–421 (2002).
D. A. Evans, H. L. McLeod, S. Pritchard, M. Tariq, and A. Mobarek. Interethnic variability in human drug responses. Drug Metab. Dispos. 29:606–610 (2001).
H. G. Xie, R. B. Kim, A. J. J. Wood, and C. M. Stein. Molecular basis of ethnic differences in drug disposition and response. Annu. Rev. Pharmacol. Toxicol. 41:815–850 (2001).
B. Chowbay, S. Zhou, and E. J. Lee. An interethnic comparison of polymorphisms of the genes encoding drug-metabolizing enzymes and drug transporters: experience in Singapore. Drug Metab. Rev. 37:327–378 (2005).
J. Y. Cho, H. S. Lim, J. Y. Chung, K. S. Yu, J. R. Kim, S. G. Shin, and I. J. Jang. Haplotype structure and allele frequencies of CYP2B6 in a Korean population. Drug Metab. Dispos. 32:1341–1344 (2004).
D. Burger, I. van der Heiden, C. la Porte, M. van der Ende, P. Groeneveld, C. Richter, P. Koopmans, F. Kroon, H. Sprenger, J. Lindemans, P. Schenk, and R. van Schaik. Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race, and CYP2B6 polymorphism. Br. J. Clin. Pharmacol. 61:148–154 (2006).
D. W. Haas, L. M. Smeaton, R. W. Shafer, G. K. Robbins, G. D. Morse, L. Labbe, G. R. Wilkinson, D. B. Clifford, R. T. D'Aquila, V. De Gruttola, R. B. Pollard, T. C. Merigan, M. S. Hirsch, A. L. George Jr., J. P. Donahue, and R. B. Kim. Pharmacogenetics of long-term responses to antiretroviral regimens containing efavirenz and/or nelfinavir: an Adult AIDS Clinical Trials Group Study. J. Infect. Dis. 192:1931–1942 (2005).
M. Rotger, S. Colombo, H. Furrer, G. Bleiber, T. Buclin, B. L. Lee, O. Keiser, J. Biollaz, L. Decosterd, and A. Telenti. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet. Genomics. 15:1–5 (2005).
S. Rodriguez-Novoa, P. Barreiro, A. Rendon, I. Jimenez-Nacher, J. Gonzalez-Lahoz, and V. Soriano. Influence of 516G>T polymorphisms at the gene encoding the CYP450-2B6 isoenzyme on efavirenz plasma concentrations in HIV-infected subjects. Clin. Infect. Dis. 40:1358–1361 (2005).
D. W. Haas, H. J. Ribaudo, R. B. Kim, C. Tierney, G. R. Wilkinson, R. M. Gulick, D. B. Clifford, T. Hulgan, C. Marzolini, and E. P. Acosta. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. Aids 18:2391–2400 (2004).
S. M. Yule, A. V. Boddy, M. Cole, L. Price, R. Wyllie, M. J. Tasso, A. D. Pearson, and J. R. Idle. Cyclophosphamide pharmacokinetics in children. Br. J. Clin. Pharmacol. 41:13–19 (1996).
A. V. Boddy, Y. Furtun, S. Sardas, O. Sardas, and J. R. Idle. Individual variation in the activation and inactivation of metabolic pathways of cyclophosphamide. J. Natl. Cancer. Inst. 84:1744–1748 (1992).
T. L. Chen, J. L. Passos-Coelho, D. A. Noe, M. J. Kennedy, K. C. Black, O. M. Colvin, and L. B. Grochow. Nonlinear pharmacokinetics of cyclophosphamide in patients with metastatic breast cancer receiving high-dose chemotherapy followed by autologous bone marrow transplantation. Cancer Res. 55:810–816 (1995).
R. Schmidt, F. Baumann, H. Hanschmann, F. Geissler, and R. Preiss. Gender difference in ifosfamide metabolism by human liver microsomes. Eur. J. Drug Metab. Pharmacokinet. 26:193–200 (2001).
H. Wang and M. Negishi. Transcriptional regulation of cytochrome p450 2B genes by nuclear receptors. Curr. Drug Metab. 4:515–525 (2003).
T. Lang, K. Klein, T. Richter, A. Zibat, R. Kerb, M. Eichelbaum, M. Schwab, and U. M. Zanger. Multiple novel nonsynonymous CYP2B6 gene polymorphisms in Caucasians: demonstration of phenotypic null alleles. J. Pharmacol. Exp. Ther. 311:34–43 (2004).
M. Spatzenegger, H. Liu, Q. Wang, A. Debarber, D. R. Koop, and J. R. Halpert. Analysis of differential substrate selectivities of CYP2B6 and CYP2E1 by site-directed mutagenesis and molecular modeling. J. Pharmacol. Exp. Ther. 304:477–487 (2003).
Acknowledgments
The authors thank Prof. Zanger, Dr. Kathrin Klein (Dr.Margarete Fischer-Bosch Institut for Klinische Pharmakologie, Stuttgart, Germany), and Prof. Mizugaki, Dr. Masahiro Hiratsuka (Department of Clinical Pharmaceutics Tohoku Pharmaceutical University, Japan) for providing DNA reference samples. This work was supported by the National Nature Science Fund of China (No. 30572231), Guangdong Nature Science Fund (no. 2003: 36622) and the National University of Singapore Academic Research Funds (nos. R-148-000-047-101 and R-148-000-067-112).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Guan, S., Huang, M., Li, X. et al. Intra- and Inter-ethnic Differences in the Allele Frequencies of Cytochrome P450 2B6 Gene in Chinese. Pharm Res 23, 1983–1990 (2006). https://doi.org/10.1007/s11095-006-9083-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9083-5