Abstract
Purpose
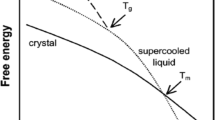
To present a calorimetry-based approach for estimating the initial (at the onset of annealing) relaxation time (τ 0) of organic amorphous solids at relatively low temperatures, and to assess the temperature where molecular mobility of the amorphous drug is reduced to a level comparable with the desired shelf-life of the product.
Materials and Methods
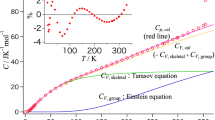
Values of τ 0 for six amorphous pharmaceutical compounds were estimated based on the nonlinear Adam–Gibbs equation. Fragility was determined from the scanning rate-dependence of the glass transition temperature (T g). The initial enthalpic and entropic fictive temperatures were obtained from the T g and the heat capacities (C p) of the amorphous and crystalline forms.
Results
At a relatively low temperature (∼40°C or more below T g), τ 0 for the different compounds varies by over an order of magnitude. For some materials, the practical storage temperature at T g − 50 K was found to be still too high to ensure long-term stability. The estimated τ 0 is highly sensitive to the fragility of the material and the C p of the crystalline and amorphous forms. Materials with high fragility or greater C p differences between crystalline and amorphous forms tend to have longer τ 0.
Conclusions
The proposed method can be used to estimate molecular mobility at relatively low temperatures without having to conduct enthalpy recovery experiments. An accurate τ 0 determination from this method relies on faithful fragility measurements.




Similar content being viewed by others
References
B. C. Hancock, S. L. Shamblin, and G. Zografi. Molecular mobility of amorphous pharmaceutical solids below their glass-transition temperatures. Pharm. Res. 12:799–806 (1995).
B. C. Hancock and G. Zografi. The relationship between the glass-transition temperature and the water-content of amorphous pharmaceutical solids. Pharm. Res. 11:471–477 (1994).
B. C. Hancock and G. Zografi. Characteristics and significance of the amorphous state in pharmaceutical systems. J. Pharm. Sci. 86:1–12 (1997).
P. Tong and G. Zografi. Effects of water vapor absorption on the physical and chemical stability of amorphous sodium indomethacin. Aaps Pharmscitech 5: Art. No. 26 (2004).
V. Andronis and G. Zografi. The molecular mobility of supercooled amorphous indomethacin as a function of temperature and relative humidity. Pharm. Res. 15:835–842 (1998).
B. C. Hancock and S. L. Shamblin. Molecular mobility of amorphous pharmaceuticals determined using differential scanning calorimetry. Thermochim. Acta 380:95–107 (2001).
S. R. Byrn, W. Xu, and A. W. Newman. Chemical reactivity in solid-state pharmaceuticals: formulation implications. Adv. Drug Deliv. Rev. 48:115–136 (2001).
K. Kawakami and M. J. Pikal. Calorimetric investigation of the structural relaxation of amorphous materials: evaluating validity of the methodologies. J. Pharm. Sci. 94:948–965 (2005).
C. Mao, S. P. Chamarthy, and R. Pinal. Time-dependence of molecular mobility during structural relaxation and its impact on organic amorphous solids: an investigation based on a calorimetric approach. Pharm. Res. 23:1906–1917 (2006).
S. L. Shamblin, B. C. Hancock, Y. Dupuis, and M. J. Pikal. Interpretation of relaxation time constants for amorphous pharmaceutical systems. J. Pharm. Sci. 89:417–427 (2000).
G. Adam and J. H. Gibbs. On temperature dependence of cooperative relaxation properties in glass-forming liquids. J. Chem. Phys. 43:139–146 (1965).
G. W. Scherer. Use of the Adam–Gibbs equation in the analysis of structural relaxation. J. Am. Ceram. Soc. 67:504–511 (1984).
I. M. Hodge. Enthalpy relaxation and recovery in amorphous materials. J. Non-Cryst. Solids 169:211–266 (1994).
K. J. Crowley and G. Zografi. The use of thermal methods for predicting glass-former fragility. Thermochim. Acta 380:79–93 (2001).
I. M. Hodge. Strong and fragile liquids—a brief critique. J. Non-Cryst. Solids 202:164–172 (1996).
S. L. Shamblin, X. L. Tang, L. Q. Chang, B. C. Hancock, and M. J. Pikal. Characterization of the time scales of molecular motion in pharmaceutically important glasses. J. Phys. Chem. B 103:4113–4121 (1999).
C. T. Moynihan, A. J. Easteal, J. Wilder, and J. Tucker. Dependence of Glass-transition temperature on heating and cooling rate. J. Phys. Chem. 78:2673–2677 (1974).
Y. Aso, S. Yoshioka, and S. Kojima. Relationship between the crystallization rates of amorphous nifedipine, phenobarbital, and flopropione, and their molecular mobility as measured by their enthalpy relaxation and 1H NMR relaxation times. J. Pharm. Sci. 89:408–416 (2000).
D. L. Zhou, G. G. Z. Zhang, D. Law, D. J. W. Grant, and E. A. Schmitt. Physical stability of amorphous pharmaceuticals: importance of configurational thermodynamic quantities and molecular mobility. J. Pharm. Sci. 91:1863–1872 (2002).
J. J. M. Ramos, R. Taveira-Marques, and H. P. Diogo. Estimation of the fragility index of indomethacin by DSC using the heating and cooling rate dependency of the glass transition. J. Pharm. Sci. 93:1503–1507 (2004).
I. Weuts, D. Kempen, K. Six, J. Peeters, G. Verreck, M. Brewster, and G. Van den Mooter. Evaluation of different calorimetric methods to determine the glass transition temperature and molecular mobility below T-g for amorphous drugs. Int. J. Pharm. 259:17–25 (2003).
Acknowledgments
The financial support from the Purdue–Michigan Joint Program on the Chemical and Physical Stability of Pharmaceutical Solids is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mao, C., Prasanth Chamarthy, S., Byrn, S.R. et al. A Calorimetric Method to Estimate Molecular Mobility of Amorphous Solids at Relatively Low Temperatures. Pharm Res 23, 2269–2276 (2006). https://doi.org/10.1007/s11095-006-9071-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9071-9