Abstract
Purpose
To determine the contribution of the lymphatics to the systemic availability of darbepoetin alfa (DA) using an established sheep model.
Materials and Methods
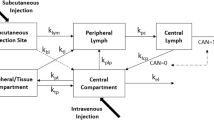
DA was administered either by intravenous (IV) injection (0.2, 0.5 or 2 μg/kg) or by subcutaneous (SC) administration (2 μg/kg) into the interdigital space of the hind leg. A SC control group was used to determine the absolute bioavailability (F sys). Cannulation of the peripheral lymphatics in a parallel SC group allowed the continuous collection of lymph draining the injection site and determination of the cumulative amount of DA absorbed via the lymphatics. Serum and lymph concentrations of DA were determined by ELISA. The fraction of the dose absorbed into the lymphatics (F lymph) relative to the fraction absorbed directly into the blood (F blood) was determined using a compartmental approach.
Results
Dose-linear pharmacokinetics was observed within the dose range investigated. The bioavailability was virtually complete following SC injection into the interdigital space (88.4 ± 15.7%). A high proportion of the administered dose was recovered in peripheral lymph (90.2 ± 4.4%) resulting in a substantial reduction in the systemic availability in lymph cannulated animals (3.7%).
Conclusion
The high recovery of DA in the peripheral lymph demonstrated near complete absorption of this recombinant protein via the lymphatics in a lymph cannulated sheep model.


Similar content being viewed by others
References
I. C. Macdougall. Novel erythropoiesis stimulating protein. Semin. Neurol. 20:375–381 (2000).
J. C. Egrie and J. K. Browne. Development and characterization of novel erythropoiesis stimulating protein (NESP). Nephrol. Dial. Transplant. 16(Suppl 3):3–13 (2001).
I. C. Macdougall, S. J. Gray, O. Elston, C. Breen, B. Jenkins, J. Browne, and J. Egrie. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J. Am. Soc. Nephrol. 10:2392–2395 (1999).
K. K. Flaharty, J. Caro, A. Erslev, J. J. Whalen, E. M. Morris, T. D. Bjornsson, and P. H. Vlasses. Pharmacokinetics and erythropoietic response to human recombinant erythropoietin in healthy men. Clin. Pharmacol. Ther. 47:557–564 (1990).
P. Veng-Pedersen, J. A. Widness, L. M. Pereira, C. Peters, R. L. Schmidt, and L. S. Lowe. Kinetic evaluation of nonlinear drug elimination by a disposition decomposition analysis. Application to the analysis of the nonlinear elimination kinetics of erythropoietin in adult humans. J. Pharm. Sci. 84:760–767 (1995).
P. Veng-Pedersen, J. A. Widness, L. M. Pereira, R. L. Schmidt, and L. S. Lowe. A comparison of nonlinear pharmacokinetics of erythropoietin in sheep and humans. Biopharm. Drug Dispos. 20:217–223 (1999).
M. Kato, H. Kamiyama, A. Okazaki, K. Kumaki, Y. Kato, and Y. Sugiyama. Mechanism for the nonlinear pharmacokinetics of erythropoietin in rats. J. Pharmacol. Exp. Ther. 283:520–527 (1997).
M. Allon, K. Kleinman, M. Walczyk, C. Kaupke, L. Messer-Mann, K. Olson, A. C. Heatherington, and B. J. Maroni. Pharmacokinetics and pharmacodynamics of darbepoetin alfa and epoetin in patients undergoing dialysis. Clin. Pharmacol. Ther. 72:546–555 (2002).
A. Heatherington, D. Robi, J. Young, and S. Baughman. Pharmacokinetic (PK) Properties of Aranesp™ Scale Allometrically. American Association of Pharmaceutical Scientists National Biotechnology Conference, San Diego, 2002.
G. Jang, R. Marino, B. Cooke, and D. Padhi. Darbepoetin Alfa (Aranesp) Pharmacokinetics is Comparable in Chronic Kidney Disease (CKD) Patients Receiving and not Receiving dialysis, in Pediatric CKD Patients, and in Healthy Adults. The American Society of Nephrology, Philadelphia, 2005.
D. Kampf, A. Kahl, J. Passlick, A. Pustelnik, K. U. Eckardt, B. Ehmer, C. Jacobs, A. Baumelou, B. Grabensee, and G. M. Gahl. Single-dose kinetics of recombinant human erythropoietin after intravenous, subcutaneous and intraperitoneal administration: preliminary results. Contrib. Nephrol. 76:106–111 (1989).
J. D. Jensen, L. W. Jensen, and J. K. Madsen. The pharmacokinetics of recombinant human erythropoietin after subcutaneous injection at different sites. Eur. J. Clin. Pharmacol. 46:333–337 (1994).
J. C. Egrie, E. Dwyer, J. K. Browne, A. Hitz, and M. A. Lykos. Darbepoetin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietin. Exp. Hematol. 31:290–299 (2003).
A. Supersaxo, W. R. Hein, and H. Steffen. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm. Res. 7:167–169 (1990).
S. A. Charman, A. M. Segrave, G. A. Edwards, and C. J. H. Porter. Systemic availability and lymphatic transport of human growth hormone administered by subcutaneous injection. J. Pharm. Sci. 89:168–177 (2000).
C. J. Porter and S. A. Charman. Lymphatic transport of proteins after subcutaneous administration. J. Pharm. Sci. 89:297–310 (2000).
S. A. Charman, D. N. McLennan, G. A. Edwards, and C. J. H. Porter. Lymphatic absorption is a significant contributor to the subcutaneous bioavailability of insulin in a sheep model. Pharm. Res. 18:1620–1626 (2001).
D. N. McLennan, C. J. H. Porter, G. A. Edwards, S. W. Martin, A. C. Heatherington, and S. A. Charman. Lymphatic absorption is the primary contributor to the systemic availability of epoetin alfa following subcutaneous administration to sheep. J. Pharmacol. Exp. Ther. 313:345–351 (2005).
C. J. H. Porter, G. A. Edwards, and S. A. Charman. Lymphatic transport of proteins after s.c. injection: implications of animal model selection. Adv. Drug Deliv. Rev. 50:157–171 (2001).
M. Gibaldi and D. Perrier. Pharmacokinetics. Marcel Dekker, New York, 1982.
D. Adams and M. McKinley. The sheep. ANZCCART News 8:1–4 (1995).
D. N. McLennan, C. J. H. Porter, and S. A. Charman. Subcutaneous drug delivery and the role of the lymphatics. Drug. Discov. Today Technol. 2:89–96 (2005).
Acknowledgments
The technical assistance of Ms. Majella Snelling is gratefully acknowledged. Financial support for this study was provided by Amgen Inc., Thousand Oaks, California.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
C s | Serum concentration |
A sc | The amount at the SC injection site |
A pl | The amount in peripheral lymph |
A e | The amount in the extravascular compartment |
Secondary parameters estimated:
Rights and permissions
About this article
Cite this article
McLennan, D.N., Porter, C.J.H., Edwards, G.A. et al. The Absorption of Darbepoetin Alfa Occurs Predominantly via the Lymphatics Following Subcutaneous Administration to Sheep. Pharm Res 23, 2060–2066 (2006). https://doi.org/10.1007/s11095-006-9064-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9064-8