Purpose
Our previous studies showed that the mRNA level of human organic anion transporter (hOAT) 3 in the kidney was correlated with the rate of elimination of an anionic antibiotic cefazolin. However, the correlation coefficient was not so high. In the present study, therefore, we enrolled more patients to examine whether additional factors were responsible for the correlation.
Methods
hOAT mRNA levels in renal biopsy specimens were quantified using the real-time polymerase chain reaction method. The elimination rates for the free fraction of cefazolin were determined in patients with various renal diseases.
Results
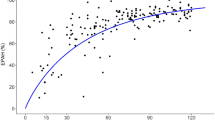
In the present study, the coefficient of correlation between the hOAT3 mRNA level and the elimination rates for the free fraction of cefazolin was not so high in the patients overall as in our previous study (r = 0.536). However, following the classification of renal diseases, a better correlation was obtained in patients with mesangial proliferative glomerulonephritis (r = 0.723). In contrast, multiple regression analyses including gender, age, and liver function did not result in any improvements in the correlation coefficients.
Conclusions
These results suggest that the hOAT3 mRNA level is a significant marker of pharmacokinetics with which to predict the rate of elimination of cefazolin in patients with mesangial proliferative glomerulonephritis.



Similar content being viewed by others
References
L. Dettli (1976) ArticleTitleDrug dosage in renal disease Clin. Pharmacokinet. 1 126–134 Occurrence Handle797495 Occurrence Handle1:STN:280:DyaE2s7hsVKlsA%3D%3D
O. Shemesh H. Golbetz J. P. Kriss B. D. Myers (1985) ArticleTitleLimitations of creatinine as a filtration marker in glomerulopathic patients Kidney Int. 28 830–838 Occurrence Handle2418254 Occurrence Handle1:STN:280:DyaL287hs1eltw%3D%3D
Y. Urakami N. Kimura M. Okuda K. Inui (2004) ArticleTitleCreatinine transport by basolateral organic cation transporter hOCT2 in the human kidney Pharm. Res. 21 976–981 Occurrence Handle15212162 Occurrence Handle10.1023/B:PHAM.0000029286.45788.ad Occurrence Handle1:CAS:528:DC%2BD2cXktlyktbY%3D
R. Hori K. Okumura A. Kamiya H. Nihira H. Nakano (1983) ArticleTitleAmpicillin and cephalexin in renal insufficiency Clin. Pharmacol. Ther. 34 792–798 Occurrence Handle6641095 Occurrence Handle1:STN:280:DyaL2c%2Fls1arsg%3D%3D Occurrence Handle10.1038/clpt.1983.251
S. E. Tett C. M. Kirkpatrick A. S. Gross A. J. McLachlan (2003) ArticleTitlePrinciples and clinical application of assessing alterations in renal elimination pathways Clin. Pharmacokinet. 42 1193–1211 Occurrence Handle14606929
J. B. Pritchard D. S. Miller (1993) ArticleTitleMechanisms mediating renal secretion of organic anions and cations Physiol. Rev. 73 765–796 Occurrence Handle8415925 Occurrence Handle1:STN:280:DyaK2c%2Fit1KgsQ%3D%3D
K. Inui M. Okuda (1998) ArticleTitleCellular and molecular mechanisms of renal tubular secretion of organic anions and cations Clin. Exp. Nephrol. 2 100–108 Occurrence Handle1:CAS:528:DyaK1cXlvFygtr4%3D
K. Inui S. Masuda H. Saito (2000) ArticleTitleCellular and molecular aspectsof drug transport in the kidney Kidney Int. 58 944–958 Occurrence Handle10972658 Occurrence Handle10.1046/j.1523-1755.2000.00251.x Occurrence Handle1:CAS:528:DC%2BD3cXmsleitr4%3D
B. C. Burckhardt G. Burckhardt (2003) ArticleTitleTransport of organic anions across the basolateral membrane of proximal tubule cells Rev. Physiol., Biochem. Pharmacol. 146 95–158 Occurrence Handle1:CAS:528:DC%2BD3sXltlKit74%3D
Y. Sakurai H. Motohashi H. Ueo S. Masuda H. Saito M. Okuda N. Mori M. Matsuura T. Doi A. Fukatsu O. Ogawa K. Inui (2004) ArticleTitleExpression levels of renal organic anion transporters (OATs) and their correlation with anionic drug excretion in patients with renal diseases Pharm. Res. 21 61–67 Occurrence Handle14984259 Occurrence Handle1:CAS:528:DC%2BD2cXkt1Onug%3D%3D
R. E. Gilbert M. E. Cooper (1999) ArticleTitleThe tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 56 1627–1637 Occurrence Handle10571771 Occurrence Handle10.1046/j.1523-1755.1999.00721.x Occurrence Handle1:STN:280:DC%2BD3c%2FktVylsg%3D%3D
M. H. Park V. D'Agati G. B. Appel C. L. Pirani (1986) ArticleTitleTubulointerstitial disease in lupus nephritis: relationship to immune deposits, interstitial inflammation, glomerular changes, renal function, and prognosis Nephron 44 309–319 Occurrence Handle3540691 Occurrence Handle1:STN:280:DyaL2s%2FptVGjsw%3D%3D Occurrence Handle10.1159/000184012
E. Alexopoulos D. Seron R. B. Hartley J. S. Cameron (1990) ArticleTitleLupus nephritis: correlation of interstitial cells with glomerular function Kidney Int. 37 100–109 Occurrence Handle1967662 Occurrence Handle1:STN:280:DyaK3c7jsVOhtw%3D%3D
H. Motohashi Y. Sakurai H. Saito S. Masuda Y. Urakami M. Goto A. Fukatsu O. Ogawa K. Inui (2002) ArticleTitleGene expression levels and immunolocalization of organic ion transporters in the human kidney J. Am. Soc. Nephrol. 13 866–874 Occurrence Handle11912245 Occurrence Handle1:CAS:528:DC%2BD38XjtFSnsb8%3D
I. Yano T. Ito M. Takano K. Inui (1997) ArticleTitleEvaluation of renal tubular secretion and reabsorption of levofloxacin in rats Pharm. Res. 14 508–511 Occurrence Handle9144740 Occurrence Handle10.1023/A:1012111902798 Occurrence Handle1:CAS:528:DyaK2sXjtVWrsbc%3D
K. A. Nath (1998) ArticleTitleThe tubulointerstitium in progressive renal disease Kidney Int. 54 992–994 Occurrence Handle9734628 Occurrence Handle10.1046/j.1523-1755.1998.00079.x Occurrence Handle1:STN:280:DyaK1cvgtlKqtQ%3D%3D
J. Floege J. Feehally (2000) ArticleTitleIgA nephropathy: recent developments J. Am. Soc. Nephrol. 11 2395–2403 Occurrence Handle11095664 Occurrence Handle1:CAS:528:DC%2BD3cXptVagsbc%3D
H. Ueo H. Motohashi T. Katsura K. Inui (2005) ArticleTitleHuman organic anion transporter hOAT3 is a potent transporter of cephalosporin antibiotics, in comparison with hOAT1 Biochem. Pharmacol 70 1104–1113 Occurrence Handle16098483 Occurrence Handle10.1016/j.bcp.2005.06.024 Occurrence Handle1:CAS:528:DC%2BD2MXpvFegsrc%3D
M. Takeda T. Sekine H. Endou (2000) ArticleTitleRegulation by protein kinase C of organic anion transport driven by rat organic anion transporter 3 (rOAT3) Life Sci. 67 1087–1093 Occurrence Handle10954042 Occurrence Handle1:CAS:528:DC%2BD3cXltlSiu74%3D
S. Soodvilai V. Chatsudthipong K. K. Evans S. H. Wright W. H. Dantzler (2004) ArticleTitleAcute regulation of OAT3-mediated estrone sulfate transport in isolated rabbit renal proximal tubules Am. J. Physiol. Renal. Physiol. 287 F1021–F1029 Occurrence Handle15238352 Occurrence Handle10.1152/ajprenal.00080.2004 Occurrence Handle1:CAS:528:DC%2BD2cXhtVaqtLzO
S. Soodvilai, S. H. Wright, W. H. Dantzler, V. Chatsudthipong. Involvement of tyrosine kinase and PI3K in the regulation ofOAT3-mediated estrone sulfate transport in isolated rabbit renal proximal tubules. Am. J. Physiol. Renal. Physiol. in press.
D. Koya G. L. King (1998) ArticleTitleProtein kinase C activation and the development of diabetic complications Diabetes 47 859–866 Occurrence Handle9604860 Occurrence Handle1:CAS:528:DyaK1cXjsFSgtrw%3D
S. H. Park H. J. Choi J. H. Lee C. H. Woo J. H. Kim H. J. Han (2001) ArticleTitleHigh glucose inhibits renal proximal tubule cell proliferation and involves PKC, oxidative stress, and TGF-beta 1 Kidney Int. 59 1695–1705 Occurrence Handle11318940 Occurrence Handle10.1046/j.1523-1755.2001.0590051695.x Occurrence Handle1:CAS:528:DC%2BD3MXjs1ehsL8%3D
J. V. Moller M. I. Sheikh (1982) ArticleTitleRenal organic anion transport system: pharmacological, physiological, and biochemical aspects Pharmacol. Rev. 34 315–358 Occurrence Handle6763702 Occurrence Handle1:STN:280:DyaL3s3itF2mug%3D%3D
G. R. Brown (1993) ArticleTitleCephalosporin–probenecid drug interactions Clin. Pharmacokinet. 24 289–300 Occurrence Handle8491057 Occurrence Handle1:CAS:528:DyaK3sXksVSjt7o%3D
T. Ishikawa A. Tsuji K. Inui Y. Sai N. Anzai M. Wada H. Endou Y. Sumino (2004) ArticleTitleThe genetic polymorphism of drug transporters: functional analysis approaches Pharmacogenomics 5 67–99 Occurrence Handle14683421 Occurrence Handle10.1517/phgs.5.1.67.25683 Occurrence Handle1:CAS:528:DC%2BD2cXhvF2rur8%3D
C. Marzolini R. G. Tirona R. B. Kim (2004) ArticleTitlePharmacogenomics of the OATP and OAT families Pharmacogenomics 5 273–282 Occurrence Handle15102542 Occurrence Handle10.1517/phgs.5.3.273.29831 Occurrence Handle1:CAS:528:DC%2BD2cXis1yjsro%3D
Y. Nishizato I. Ieiri H. Suzuki M. Kimura K. Kawabata T. Hirota H. Takane S. Irie H. Kusuhara Y. Urasaki A. Urae S. Higuchi K. Otsubo Y. Sugiyama (2003) ArticleTitlePolymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmacokinetics Clin. Pharmacol. Ther. 73 554–565 Occurrence Handle12811365 Occurrence Handle10.1016/S0009-9236(03)00060-2 Occurrence Handle1:CAS:528:DC%2BD3sXkt1Ortbg%3D
M. Niemi E. Schaeffeler T. Lang M. F. Fromm M. Neuvonen C. Kyrklund J. T. Backman R. Kerb M. Schwab P. J. Neuvonen M. Eichelbaum K. T. Kivisto (2004) ArticleTitleHigh plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1) Pharmacogenetics 14 429–440 Occurrence Handle15226675 Occurrence Handle1:CAS:528:DC%2BD2cXlt1Cktrk%3D
S. H. Cha T. Sekine J. I. Fukushima Y. Kanai Y. Kobayashi T. Goya H. Endo (2001) ArticleTitleIdentification and characterization of human organic anion transporter 3 expressing predominantly in the kidney Mol. Pharmacol. 59 1277–1286 Occurrence Handle11306713 Occurrence Handle1:CAS:528:DC%2BD3MXjt1aqtbo%3D
H. Motohashi Y. Uwai K. Hiramoto M. Okuda K. Inui (2004) ArticleTitleDifferent transport properties between famotidine and cimetidine by human renal organic ion transporters (SLC22A) Eur. J. Pharmacol. 503 25–30 Occurrence Handle15496291 Occurrence Handle10.1016/j.ejphar.2004.09.032 Occurrence Handle1:CAS:528:DC%2BD2cXovFagur0%3D
Y. Uwai R. Taniguchi H. Motohashi H. Saito M. Okuda K. Inui (2004) ArticleTitleMethotrexate–loxoprofen interaction: involvement of human organic anion transporters hOAT1 and hOAT3 Drug Metab. Pharmacokinet. 19 369–374 Occurrence Handle15548848 Occurrence Handle10.2133/dmpk.19.369 Occurrence Handle1:CAS:528:DC%2BD2MXhtVyhsA%3D%3D
M. Takeda S. Khamdang S. Narikawa H. Kimura Y. Kobayashi T. Yamamoto S. H. Cha T. Sekine H. Endou (2002) ArticleTitleHuman organic anion transporters and human organic cation transporters mediate renal antiviral transport J. Pharmacol. Exp. Ther. 300 918–924 Occurrence Handle11861798 Occurrence Handle10.1124/jpet.300.3.918 Occurrence Handle1:CAS:528:DC%2BD38XitVemurY%3D
Acknowledgments
This work was supported by a grant-in-aid for Comprehensive Research on Aging and Health from the Ministry ofHealth and Welfare of Japan (H15-Choju-006), by a grant-in-aid for Scientific Research from the Ministry of Education,Culture, Sports, Science, and Technology of Japan, by agrant-in-aid from the Japan Research Foundation for Clinical Pharmacology, and by the 21st Century COE program“KnowledgeInformation Infrastructure for Genome Science.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakurai, Y., Motohashi, H., Ogasawara, K. et al. Pharmacokinetic Significance of Renal OAT3 (SLC22A8) for Anionic Drug Elimination in Patients with Mesangial Proliferative Glomerulonephritis. Pharm Res 22, 2016–2022 (2005). https://doi.org/10.1007/s11095-005-8383-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-005-8383-5