Abstract
Purpose.
To investigate the mechanism by which polyethylene glycol (PEG) conjugation (PEGylation) prevents the acylation of octreotide by poly(d,l-lactide-co-glycolide) (PLGA).
Methods.
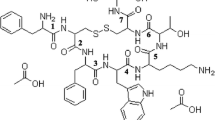
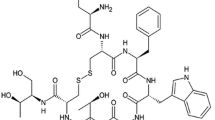
Octreotide was chemically modified by reaction with succinimidyl propionate-monomethoxy PEG. Each PEGylated octreotide species with different PEG number and modified position was separated by reversed-phase high-performance liquid chromatography (RP-HPLC) and characterized by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) with endoproteinase Lys-C digestion. Acylation of octreotide and PEGylated octreotides was observed with hydrophobic and hydrophilic PLGA.
Results.
Two mono- and one di-PEGylated octreotides were separated by RP-HPLC. MALDI-TOF MS of the PEGylated products after Lys-C digestion at different pH revealed that the two mono-PEGylated octreotides were modified at the N-terminus and Lys5 residue, respectively. The interaction of octreotide with PLGA involved an initial adsorption followed by acylation and the subsequent release of octreotide and acylated octreotide. The initial adsorption of octreotide was dependent on the acidity of PLGA. PEGylation of octreotide significantly inhibited the initial adsorption and acylation by PLGA. In particular, the acylation could be completely prevented by mono-PEGylation at the N-terminus of octreotide.
Conclusions.
This study shows that the N-terminus of octreotide is the preferred PEGylation site to prevent acylation in degrading PLGA microspheres. The mono-N-terminally PEGylated octreotide may possibly serve as a new source for somatostatin microsphere formulation.
Similar content being viewed by others
References
1. W. R. Gombotz and D. K. Pettit. Biodegradable polymers for protein and peptide drug delivery. Bioconjug. Chem. 6:332–351 (1995).
2. B. H. Woo, B. F. Fink, R. Page, J. A. Schrier, Y. W. Jo, G. Jiang, M. DeLuca, H. C. Vasconez, and P. P. DeLuca. Enhancement of bone growth by sustained delivery of recombinant human bone morphogenetic protein-2 in a polymeric matrix. Pharm. Res. 18:1747–1753 (2001).
3. B. H. Woo, K.-H. Na, B. A. Dani, G. Jiang, B. C. Thanoo, and P. P. DeLuca. In vitro characterization and in vivo testosterone suppression of 6-month release poly(D,L-lactide) leuprolide microspheres. Pharm. Res. 19:546–550 (2002).
4. M. van de Weert, W. E. Hennink, and W. Jiskoot. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm. Res. 17:1159–1167 (2000).
5. A. Brunner, K. Mäder, and A. Göpferich. pH and osmotic pressure inside biodegradable microspheres during erosion. Pharm. Res. 16:847–853 (1999).
6. K. Fu, D. W. Pack, A. M. Klibanov, and R. Langer. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm. Res. 17:100–106 (2000).
7. A. Rothen-Weinhold, N. Oudry, K. Schwach-Abdellaoui, S. Frutiger-Hughes, G. J. Hughes, D. Jeannerat, U. Burger, K. Besseghir, and R. Gurny. Formation of peptide impurities in polyester matrices during implant manufacturing. Eur. J. Pharm. Biopharm. 49:253–257 (2000).
8. A. Lucke, J. Kiermaier, and A. Göpferich. Peptide acylation by poly(α-hydroxy esters). Pharm. Res. 19:175–181 (2002).
9. D. H. Na, Y. S. Youn, S. D. Lee, M. W. Son, W. B. Kim, P. P. DeLuca, and K. C. Lee. Monitoring of peptide acylation inside degrading PLGA microspheres by capillary electrophoresis and MALDI-TOF mass spectrometry. J. Control. Rel. 92:291–299 (2003).
10. A. Lucke, E. Fustella, J. Teßmar, A. Gazzaniga, and A. Göpferich. The effect of poly(ethylene glycol)-poly(D,L-lactic acid) diblock copolymers on peptide acylation. J. Control. Rel. 80:157–168 (2002).
11. S. W. Lamberts, A. J. van der Lely, W. W. de Herder, and L. J. Hofland. Octreotide. N. Engl. J. Med. 334:246–254 (1996).
12. S. B. Murty, J. Goodman, B. C. Thanoo, and P. P. DeLuca. Identification of chemically modified peptide from poly(D,L-lactide-co-glycolide) microspheres under in vitro release conditions. AAPS PharmSciTech. 4:article 50 (2003).
13. D. H. Na, Y. S. Youn, E. J. Park, J. M. Lee, O. R. Cho, K. R. Lee, S. D. Lee, S. D. Yoo, P. P. DeLuca, and K. C. Lee. Stability of PEGylated salmon calcitonin in nasal mucosa. J. Pharm. Sci. 93:256–261 (2004).
14. D. H. Na, S. B. Murty, K. C. Lee, B. C. Thanoo, and P. P. DeLuca. Preparation and stability of PEGylated octreotide for application to microsphere delivery. AAPS PharmSciTech. 4:article 72 (2003).
15. K. C. Lee, S. C. Moon, M. O. Park, J. T. Lee, D. H. Na, S. D. Yoo, H. S. Lee, and P. P. DeLuca. Isolation, characterization and stability of positional isomers of mono-PEGylated salmon calcitonins. Pharm. Res. 16:818–823 (1999).
16. D. H. Na, E. J. Park, Y. S. Youn, B. W. Moon, Y. W. Jo, S. H. Lee, W. B. Kim, Y. Sohn, and K. C. Lee. Sodium dodecyl sulfate-capillary gel electrophoresis of polyethylene glycolylated interferon alpha. Electrophoresis 25:476–479 (2004).
17. A. M. Felix, Y. A. Lu, and R. M. Campbell. Pegylated peptides. IV. Enhanced biological activity of site-directed pegylated GRF analogs. Int. J. Pept. Protein Res. 46:253–264 (1995).
18. M. Morpurgo, C. Monfardini, L. J. Hofland, M. Sergi, P. Orsolini, J. M. Dumont, and F. M. Veronese. Selective alkylation and acylation of alpha and epsilon amino groups with PEG in a somatostatin analogue: tailored chemistry for optimized bioconjugates. Bioconjug. Chem. 13:1238–1243 (2002).
19. D. H. Na, M. O. Park, S. Y. Choi, Y. S. Kim, S. S. Lee, S. D. Yoo, H. S. Lee, and K. C. Lee. Identification of the modifying sites of mono-PEGylated salmon calcitonins by capillary electrophoresis and MALDI-TOF mass spectrometry. J. Chromatogr. B 754:259–263 (2001).
20. D. H. Na and K. C. Lee. Capillary electrophoretic characterization of PEGylated human parathyroid hormone with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Biochem. 331:322–328 (2004).
21. J. A. Schrier and P. P. DeLuca. Recombinant human bone morphogenetic protein-2 binding and incorporation in PLGA microsphere delivery systems. Pharm. Dev. Technol. 4:611–621 (1999).
22. D. H. Na, Y. S. Youn, and K. C. Lee. Optimization of the PEGylation process of a peptide by monitoring with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 17:2241–2244 (2003).
23. S. S. Wong. Chemistry of Protein Conjugation and Cross-Linking, CRC Press, Boca Raton, FL, 1991.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Na, D., DeLuca, P. PEGylation of Octreotide: I. Separation of Positional Isomers and Stability Against Acylation by Poly(D,L-lactide-co-glycolide). Pharm Res 22, 736–742 (2005). https://doi.org/10.1007/s11095-005-2589-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-005-2589-4