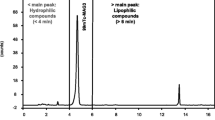
A search for a modern method to determine the radiochemical purity of an 111In-oxine radiopharmaceutical composition resulted in the selection of a chromatography system that could perform quality control rapidly, consistently, and more accurately than an extraction method. The simplicity of the method made it accessible and convenient for performing quality control in medical institutions immediately before labeling leukocytes and stem cells.



Similar content being viewed by others
References
I. O. Tomashevskii, L. D. Soshin, A. I. Luchshev, et al., Radiol.-prakt., 2, 40 – 42 (2004).
M. Roca, E. F. J. de Vries, F. Jamar, et al., Eur. J. Nucl. Med. Mol. Imaging, 37(4), 835 – 841 (2010).
J. P. Horcajada, M. Gutierrez-Cuadra, I. Martinez-Rodriguez, et al., Eur. J. Clin. Microbiol., 31(3), 237 – 242 (2012).
R. D. Dhekne, S. N. Chatziioannou, W. H. Moore, et al., Am. J. Gastroenterol., 95(8), 1983 – 1989 (2000).
K. Takahashi, M. Ohyanagi, K. Ikeoka, et al., J. Nucl. Cardiol., 8(2), 165 – 170 (2001).
A. V. Meleshina, E. I. Cherkasova, M. V. Shirmanova, et al., Sovrem. Tekhnol. Med., 7(4), 174 – 188 (2015).
G. E. Kodina, E. A. Lyamtseva, A. O. Malysheva, et al., in: Abstracts of Papers of the VIth Troitsk Conference on Medical Physics and Innovation in Medicine [in Russian], Troitsk (2014), pp. 679 – 680.
US Pharmacopoeia, USP 38, USP Monograph “Indium In-111 Oxyquinoline Solution” (2016).
G. E. Kodina, E. A. Lyamtseva, A. O. Malysheva, et al., in: Abstracts of Papers of the International Scientific-Practical Conference RADIOFARMA-2015 [in Russian], Moscow (2015), p. 44.
European Pharmacopoeia 8.0 (vol. 1), Monograph “Indium (111In) Oxine Solution” (2014), p. 1066.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 52, No. 10, pp. 54 – 58, October, 2018.
Rights and permissions
About this article
Cite this article
Taratonenkova, N.A., Lyamtseva, E.A., Malysheva, A.O. et al. Quality Control Method for 111In-Oxine Radiopharmaceutical Composition. Pharm Chem J 52, 868–872 (2019). https://doi.org/10.1007/s11094-019-1918-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-1918-6