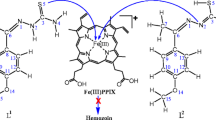
There is a method to employ aberrant levels of mobile ferrous iron (Fe(II)) for selective drug delivery in vivo. This approach makes use of a 1,2,4-trioxolane moiety, which serves as an Fe(II)- sensitive “trigger,” making drug release contingent on Fe(II)-promoted trioxolane fragmentation. In this study we focused on the prototype drug conjugate of 1,2,4-trioxolane ring joined via a traceless linker to drug species, which is conjugated to the linker via a free amine or alcohol function to form a complex.We studied four complexes formed of different drugs with various linkers. The structure and physicochemical properties of complexes were studied using the Hartree – Fock method with 6-311++G** basis set. The influence of the oxalan ring and various drugs on the properties of complexes has been studied. Geometric parameter, energies, nuclear chemical shielding constants, direction of dipole moment vector, partition coefficients, electric polarizabilities, and other physicochemical properties of these compounds have been calculated.








Similar content being viewed by others
References
R. Karaman and H. Hallak, Chem. Biol. Drug Des., 76(4), 350 – 360 (2010).
A. T. Peterson, BMC Infect. Dis., 9, 59 (2009).
A. Hitani, T. Nakamura, H. Ohtomo, et al., J. Infect. Chemother., 12(5),277 – 282 (2006).
H. J. Vial, S.Wein, C. Farenc, et al., Proc. Natl. Acad. Sci. USA, 101(43), 15458 – 15463 (2004).
M. W. Hentze, M. U. Muckenthaler, B. Galy, and C. Camaschella, Cell, 142(1), 24 – 38 (2010).
J. L. Vennerstrom et al., Nature, 430(7002), 900 – 904 (2004).
N. Valecha et al., Clin. Infect. Dis., 51(6), 684 – 691 (2010).
J. J. Moehrle et al., Brit. J. Clin. Pharmacol., 75(2), 524 – 537 (2013).
C. L. Hartwig et al., J. Med. Chem., 54(23), 8207 – 8213 (2011).
C. L. Hartwig et al., Biochem. Pharmacol. 77(3), 322 – 336 (2009).
M. A. Fügi, S. Wittlin, Y. Dong, and J. L. Vennerstrom, Antimicrob. Agents Chemother., 54(3), 1042 – 1046 (2010).
D. J. Creek et al., Antimicrob. Agents Chemother., 52(4), 1291 – 1296 (2008).
A. E. Mercer et al., J. Biol. Chem., 282(13), 9372 – 9382 (2007).
U. Eckstein-Ludwig et al., Nature, 424(6951), 957 – 961 (2003).
R. K. Haynes et al., Chem. Med. Chem., 7(12), 2204 – 2226 (2012).
P. M. O’Neill, et al., Angew. Chem. Int. Ed. (Engl.), 43(32), 4193 – 4197 (2004).
M. Klemba, I. Gluzman, and D. E. Goldberg, J. Biol. Chem., 279(41), 43000 – 43007 (2004).
E. Deu et al., Chem. Biol., 17(8), 808 – 819 (2010).
S. Arastu-Kapur et al., Nat. Chem. Biol., 4(3), 203 – 213 (2008).
E. Deu et al., Proc. Natl. Acad. Sci. USA, 110(45), 18244 – 18249 (2013).
B. Spangler et al., J. Med. Chem., 59, 11161 – 11170 (2016).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayat, Z., Gholizadeh, A. Calculations of Geometric Parameters and Physicochemical Properties of Complexes Formed of FE(II)-Reactive 1,2,4-Trioxolane Ring and Some Anti-Malaria Drugs Via Traceless Linker. Pharm Chem J 53, 411–418 (2019). https://doi.org/10.1007/s11094-019-02012-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-02012-0