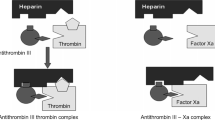
The development of efficient production of unfractionated heparin (UFH) preparations, which are widely used in clinical practice, is currently a critical problem. According to WHO guidelines, specific and selective methods for monitoring heparin activity in preparations and substances must be used in the quality assurance system for producing pharmaceutical preparations. A clotting method for measuring UFH activity in its preparations and substances according to the coagulation method recommended by the State Pharmacopoeia of the Russian Federation was developed based on the created test system. The method was evaluated using the main analytical characteristics. The results obtained from the developed test were shown to be comparable with those from UFH activity determinations using the chromogenic method recommended by the State and European pharmacopoeias. The established accuracy, sensitivity, and precision allowed the validated coagulation method and the created test system to be recommended for inclusion in UFH process monitoring.

Similar content being viewed by others
References
D. M. Zubairov, Molecular Bases of Blood Clotting and Thrombus Formation [in Russian], FEN, Kazan (2000), pp. 219 – 240.
R. U. Kolmen, Disruption of Thrombin-formation Reactions [in Russian], Meditsina, Moscow (1988), pp. 175 – 207.
M. Johnston, in: Quality in Laboratory Hemostasis and Thrombosis, S. Kitchen, J. D. Olson, and F. E. Preston (eds.), Wiley-Blackwell (2009), pp. 170 – 178.
J. Choay, M. Petitou, J. C. Lormeau, et al., Biochem. Biophys. Res. Commun., 116, 492 – 499 (1983).
S. D. Niles, R. G. Sutton, J. Ploessl, et al., J. Extracorpor. Technol., 7, 197 – 200 (1995).
A. G. Turpie, A. S. Gallus, and J. A. Hoek, N. Engl. J. Med., 344, 619 – 625 (2001).
European Pharmacopoeia 8.0. 2.7.5, Assay of Heparin, 01 /2008:20705 (2013), pp. 237 – 238.
WHO Expert Committee on Specification for Pharmaceutical Preparations, Thirty-second report, WHO, Geneva (1992), pp. 117 – 121.
State Pharmacopoeia of the RF, XIIIth Ed., Quantitative Determination of Heparin. Clotting Method [in Russian], GPM 1.8.2.0003 (2015), pp. 921 – 922.
European Pharmacopoeia, 8.3. 2.7.5, Assay of Heparin, 01 /2015:20705 (2015), p. 4187.
I. Gouin-Thibaut, I. Martin-Toutain, E. Peynaud-Debayle, et al., Thromb. Res., 129, 666 – 667 (2012).
A. M. van den Besselaar, A. Sturk, and G. L. Reijnierse, Thromb. Res., 107, 235 – 240 (2002).
S. Kitchen, I. Jennings, and T. Woods, J. Clin. Pathol., 49, 10 – 14 (1996).
K. Cuker, A. Raby, K. A. Moffat, et al., Thromb. Haemostasis, 104, 837 – 844 (2010).
GOST R 53022.2 – 2008, “Rules for estimating analytical sensitivity of clinical laboratory study methods,” Standartinform, Moscow (2009), p. 6.
V. I. Dvorkin, V. N. Malakhov, E. V. Zaikin, et al., Zh. Anal. Khim., 40, 2117 – 2124 (1985).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 52, No. 9, pp. 49 – 51, September, 2018.
Rights and permissions
About this article
Cite this article
Berkovskii, A.L., Sergeeva, E.V. & Suvorov, A.V. Unfractionated Heparin Activity Testing in Preparations and Substances. Pharm Chem J 52, 796–798 (2018). https://doi.org/10.1007/s11094-018-1902-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1902-6