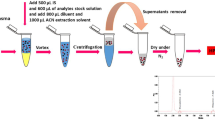
A simple and sensitive HPLC method was developed and validated to detect an anti-glioma drug (temozolomide) in rat plasma and brain. The drug and internal standard (metronidazole) were eluted at proper retention times using BDS C18 column and selected mobile phase (ammonium formate – acetonitrile). The proposed method showed specificity and linearity with R2 values of 0.9964 and 0.9978 in plasma and brain, respectively. Other parameters such as intraday and interday precision were found to be within acceptable limits. The LOD and LOQ were 84 and 255 ng/mL, respectively. The drug recovery from the spiked plasma varied from 43 to 52%. Furthermore, stability of the method was evaluated and it was found that the drug exhibited good stability except at room temperature. The percentage recovery at room temperature after 24 h was within 69 – 73%; however, the drug was stable up to 15 days at –70°C.




Similar content being viewed by others
References
L. Reyderman, P. Statkevich, C. M. Thonoor, et al., Xenobiotica, 34(5), 487 – 500 (2004).
Product Monograph: Temodal, Merck Canada Inc., October 12 (2012).
D. Jain, R. Athawale, A. Bajaj, et al., J. Chromatogr. B, Anal. Technol. Biomed. Life Sci., 970, 86 – 94 (2014).
H. Kim, P. Likhari, D. Parker, et al., J. Pharm. Biomed. Anal., 24, 461 – 468 (2001).
E. Gilant, M. Kaza, A. Szlagowska, et al., Acta Polon. Pharm., 69(6), 1347 – 1355 (2012).
M. Andrasi, B. Torzsok, A. Klekner, et al., J. Chromaogrt. B, Anal. Technol. Biomed. Life sci., 879(23), 2229 – 2233 (2011).
ICH Harmonized Tripartite Guideline and Methodology Q2 (R1), ICH Harmonized Tripartite Guideline, Validation of analytical procedures: Text and Methodology Q2 (R1), International Conference on Harmonization. Geneva, Switzerland (2005).
H. P. Chhetri, P. Thapa, and A. V. Schepdael, Saudi Pharm. J., 22(5), 483 – 487 (2014).
Z. Attari, L. Kumar, C. M. Rao, et al., Lat. Am. J. Pharm., 35, 967 – 971 (2016).
E. S. Grumbach, D. M. Wagrowski-Diehl, and J. R. Mazzeo, LC-GC North Am., 22(10), 1010 – 1023 (2004).
Acknowledgments
The authors are thankful to Ind-Swift Laboratories Ltd. for providing temozolomide as gift sample. The authors are thankful to Manipal University for providing the opportunity to carry out the present study. The authors also thank UGC-MANF for awarding the fellowship.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Attari, Z., Kumar, L., Mallikarjuna Rao, C. et al. Reversed-Phase HPLC Method for Determination of Temozolomide in Rat Plasma and Brain: Simple, Sensitive and Robust Method. Pharm Chem J 52, 266–270 (2018). https://doi.org/10.1007/s11094-018-1804-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1804-7